X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18
Nikola Pavletich (Memorial Sloan-Kettering Institute)
The growth of the human cell is controlled by a set of proteins that act
as molecular switches. These switches are turned on or off by several
other proteins and ensure that the cell grows only when appropriate. In
cancer, genetic alterations often cause the deregulation of these
switches and lead to the uncontrolled growth of the cell.
The Cyclin-dependent kinase proteins (CDKs) play a central role in
coordinating the growth and proliferation of the eukaryotic cell. At the
molecular level, CDKs act as the switches that control the cell’s
progression through the cell division cycle, and also serve to process
diverse growth-regulatory signals. They are turned on by the binding of
Cyclin proteins and by modification through phosphorylation. They are
turned off by the binding of the CIP or INK4 proteins.
At CHESS, crystallographic studies of CDKs in five different complexes
have revealed the mechanisms by which these regulatory processes control
the CDK switches. All of these mechanisms involve conformational changes
in and around the catalytic cleft of the CDK, indicating that CDKs have
evolved an intrinsic conformational flexibility. This flexibility may be
central to their ability to switch states in response to a diverse range
of growth-regulatory signals.
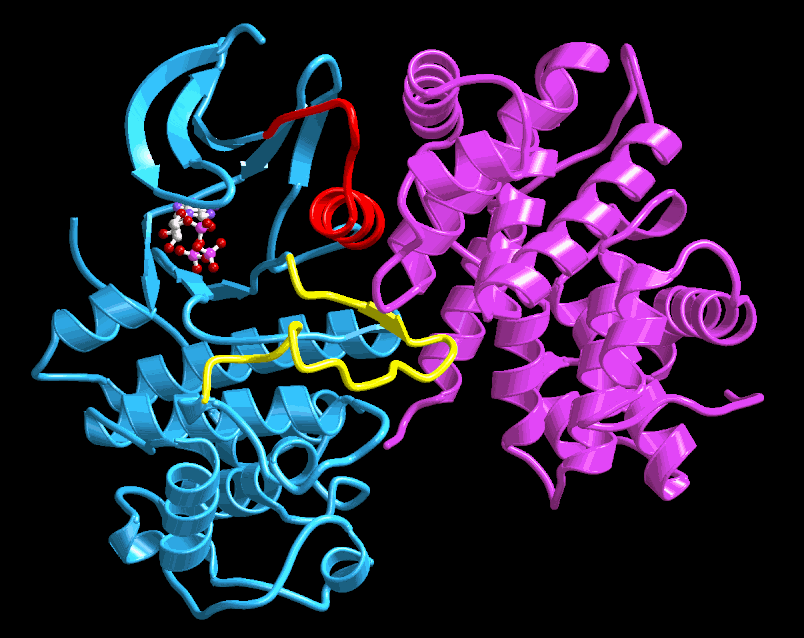
This figure shows a molecular ribbon model of how the
CDK switches (blue)
are turned on by CYCLIN proteins (magenta, right) and turned off by
CKI proteins (not shown). Genetic alterations in the genes for CDKs,
CYCLINs and CKIs are among the most frequent events in cancer.
