X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18

John Ferguson
Contact: A. Woll (aw30@cornell.edu) and E. Fontes (ef11@cornell.edu)
Ithaca, New York. G-line scientist Arthur Woll organized a night of science, food and discussion for Cornell graduate students who do X-ray work at CHESS, the Cornell High Energy Synchrotron Source. G-line is a set of three high-intensity X-ray stations that were built by graduate students a few years ago, and continue to be maintained by students to serve the research needs of the Cornell Community. This symposium is held twice a year, giving the CHESS community an update on the accomplishments of the local faculty groups and student projects. This sixth meeting in the series was held Tuesday evening, January 23rd, 2007 in the Commons area of the Wilson Laboratory. Pizza, soft drinks, and coffee helped sustain the agenda!
Four students each gave 20-minute presentations. Kurt Andresen started the night with “Anomalous Small-angle X-ray Scattering (ASAXS) Study of Multivalent Ion-DNA Interactions.” Kurt is a member of the biophysics graduate group of Lois Pollack, professor in the Applied and Engineering Physics (A&EP) department. He opened by noting that, despite its critical importance to biological functions, little experimental work directly explores ion-DNA interactions. In earlier work, Kurt and collaborators showed, using ASAXS, that a theory based on the Poisson-Boltzmann (PB) equation successfully explains the exchange between mono- and divalent cations surrounding DNA as the relative concentration of such ions is varied [1-3]. Kurt then presented preliminary data from ongoing experiments exploring the limits of the Poisson-Boltzmann equation as well as other theories.
John Ferguson followed with “Determining Growth Modes of SrTiO3 (001) Homoepitaxy via Pulsed Laser Deposition (PLD) Using In-situ X-ray Reflectivity.” John works in the group of Joel Brock, professor and chair of the A&EP department. Homoepitaxial SrTiO3 thin films were grown on single-crystal SrTiO3 (001) via PLD, a process where a plume of material is rapidly ejected from the source by an energetic laser beam, and directed onto a heated substrate. The growth was monitored in real time by in-situ X-ray reflectivity measurements at the specular anti-Bragg position (Figure 1). John described measurements designed to map the transition between step-flow, layer-by-layer, and 3D growth as a function of substrate miscut. A cross-over from layer-by-layer to step-flow growth occurs when the distance between substrate steps approaches 20 nanometers, closely matching the distance between nucleating islands found using similar growth parameters in earlier work [4]. Both distances are determined by the mean diffusion length of adatoms on the surface following each pulse. An interesting feature of PLD as compared to traditional growth techniques is that, even when growth conditions favor the eventual onset of 3D growth, the early-time anti-Bragg reflectivity oscillates as in layer-by-layer growth. A similar transition at a constant laser repetition rate occurs as the surface temperature is decreased.
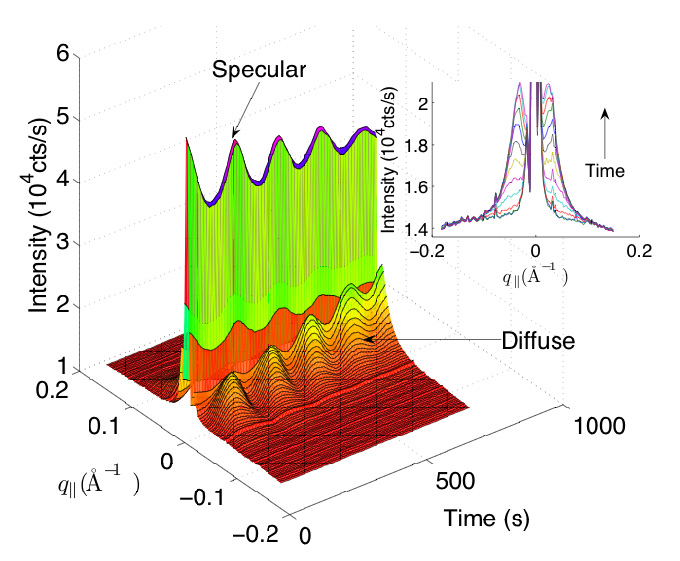
Figure 1. X-ray intensity
measured around (0,0,0.2) during SrTiO3 homoepitaxy at T=970 K, PO2 = 10-5 Torr and layer pulse frequency = 0.1 Hz. An absorber
attenuated the specular beam. Inset: Diffuse
intensity during deposition of first half-monolayer.
Each curve represents the integrated signal following
a pulse. Reproduced from [4].
After a ten-minute “half-time” break, student Yi Liu presented “Surface Characterization of Novel Electrocatalysts for Fuel Cell Applications.” Yi is a chemist studying with Héctor Abruña, professor and chair in the Chemistry and Chemical Biology department and director of the Cornell Fuel Cell Institute (http://abruna.chem.cornell.edu/). Yi opened by discussing how ordered intermetallic compounds such as PtBi and PtPb are promising candidate electrocatalysts for the oxidation of small organic molecules, and have great potential applications in fuel cells [5,6]. Because these reactions take place at the electrode/solution interface, understanding the surface structure of electrodes and how they change during electrochemical activity should provide insight into improving catalytic performance. His group built an in-situ experimental cell and preliminary data was consistent with ex-situ results (Figure 2). Measurements on PtBi and PtPb electrodes suggest that the non-platinum metal leaches from the electrode surface when subjected to cleaning and activation treatments. For single crystal electrode surfaces, metallic Pt domains were formed when the electrode was subjected to 0.8V. The audience was engaged in a discussion of the relative orientation and commensurability of the overlayer versus substrate. Further results indicate that the leaching process is suppressed when formic acid, rather than sulfuric acid, was used as the electrolyte.

Figure 2.
Electrochemical cell for in-situ X-ray
characterization
of catalyst surfaces. (Abruña Group)
Last but not least, Hitesh Arora presented “Silicon Nanostructures from Block Copolymer Derived Thin Films.” Hitesh is a chemical engineer in the research group of Uli Wiesner in Cornell’s Materials Science and Engineering department. The focus of his research is fabricating organic-inorganic hybrid thin films and their application to silicon nanostructures. To this end a patterned aluminosilicate template is formed by spin-coating a self-assembled mixture of PI-b-PEO block copolymers with aluminosilicate sol nanoparticles onto a silicon substrate and calcining at high temperatures [7,8]. The resulting honeycomb-like aluminosilicate films are typically 15-20 nanometers thick over an entire 100 millimeter silicon substrate and are characterized using AFM, SEM and GISAXS to obtain information on pattern quality. Next, amorphous silicon is deposited on the calcined film, and laser-annealed to crystallize in the pores of the amorphous aluminosilicate film. Finally, the aluminosilicate is removed using hydrofluoric acid. Subsequent TEM analysis shows that the deposited nano-pillars crystallize in registry with the atoms of the substrate (Figure 3). This method may become a cost-effective and rapid way to realize silicon nanostructures down to the tens of nanometers regime without the use of traditional lithographic techniques.
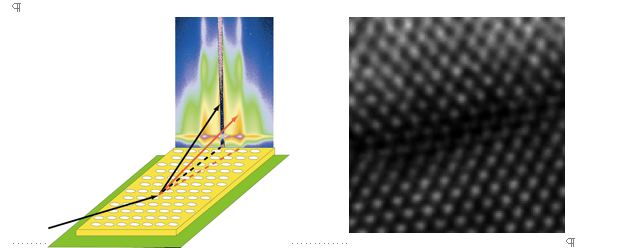
Figure 3. (left) Schematic geometry of the GISAXS data
collection from nanopatterned thin films (from D.
Smilgies). (right) SEM image of registry of silicon
atoms in pillars, top, with substrate below. (Wiesner
group).
At the evening’s conclusion, many audience members commented on the high quality of the presentations and discussion, in addition to appreciating that the evening stayed on schedule! The students and faculty all expressed hopes for the upcoming year and looked forward to hearing updated reports at the next symposium in early fall.
References:
[1] Andresen K, Das R, Park HY, Smith H, Kwok LW, Lamb JS, Kirkland EJ, Herschlag D, Finkelstein KD, Pollack L. Spatial distribution of competing ions around DNA in solution. Physical Review Letters 2004;93 (24):-.
[2] Das R, Kwok LW, Millett IS, Bai Y, Mills TT, Jacob J, Maskel GS, Seifert S, Mochrie SGJ, Thiyagarajan P, Doniach S, Pollack L, Herschlag D. The fastest global events in RNA folding: Electrostatic relaxation and tertiary collapse of the tetrahymena ribozyme. Journal of Molecular Biology 2003;332 (2):311-9.
[3] Das R, Mills TT, Kwok LW, Maskel GS, Millett IS, Doniach S, Finkelstein KD, Herschlag D, Pollack L. Counterion distribution around DNA probed by solution X-ray scattering. Physical Review Letters 2003;90 (18):-.
[4] Fleet A, Dale D, Woll AR, Suzuki Y, Brock JD. Multiple time scales in diffraction measurements of diffusive surface relaxation. Physical Review Letters 2006;96 (5):-.
[5] Blasini DR, Rochefort D, Fachini E, Alden LR, DiSalvo FJ, Cabrera CR, Abruna HD. Surface composition of ordered intermetallic compounds PtBi and PtPb. Surface Science 2006;600 (13):2670-80.
[6] Casado-Rivera E, Volpe DJ, Alden L, Lind C, Downie C, Vazquez-Alvarez T, Angelo ACD, DiSalvo FJ, Abruna HD. Electrocatalytic activity of ordered intermetallic phases for fuel cell applications. Journal of the American Chemical Society 2004;126 (12):4043-9.
[7] Templin M, Franck A, DuChesne A, Leist H, Zhang YM, Ulrich R, Schadler V, Wiesner U. Organically modified aluminosilicate mesostructures from block copolymer phases. Science 1997;278 (5344):1795-8.
[8] Du P, Li MQ, Douki K, Li XF, Garcia CRW, Jain A, Smilgies DM, Fetters LJ, Gruner SM, Wiesner U, Ober CK. Additive-driven phase-selective chemistry in block copolymer thin films: The convergence of top-down and bottom-up approaches. Advanced Materials 2004;16 (12):953-+.