X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18
By Alex Mellnik* - Oct. 6, 2010
Huimeng Wu, Feng Bai, Zaicheng Sun, Raid E. Haddad, Daniel M. Boye, Zhongwu Wang, Jian Yu Huang, and Hongyou Fan. Nanostructured Gold Architectures Formed through High Pressure-Driven Sintering of Spherical Nanoparticle Arrays. Journal of the American Chemical Society 132, 12826-12828 (2010).
Gold is famous for its excellent electrical properties and resistance to corrosion, but has also recently attracted much attention for its potential as a catalyst [1]. While platinum group materials have long been used as catalysts, it was not until the mid-1980s that a group of Japanese researchers discovered that gold is an excellent catalyst for converting carbon monoxide to carbon dioxide [2]. Since then, many other uses for gold catalysts have been found including hydrogen gas production, polymer synthesis and air purification [3].
In these applications, the activity of gold increases roughly with its surface area. Currently, many applications use gold nanoparticles, several nanometers in diameter, deposited on a support material. While the use of a support material provides a structural framework for the nanoparticles, the support materials can interfere with the catalytic action and decrease the active surface area per weight. To increase the surface area even further and remove the need for other materials, scientists hope to replace the support material with even more gold to form a self-supporting, porous gold network with nanometer-scale textures.
One common method of forming these networks is to start with an alloy of gold and a second metal, such as silver, and then to chemically etch away the other metal [4]. This method has been used to create networks with features as small as 10 nanometers, although the resulting surface-area per weight is not significantly improved over supported nanoparticles and the process requires harsh chemical etchants.
A schematic diagram of the experiment with
scanning electron microscopy
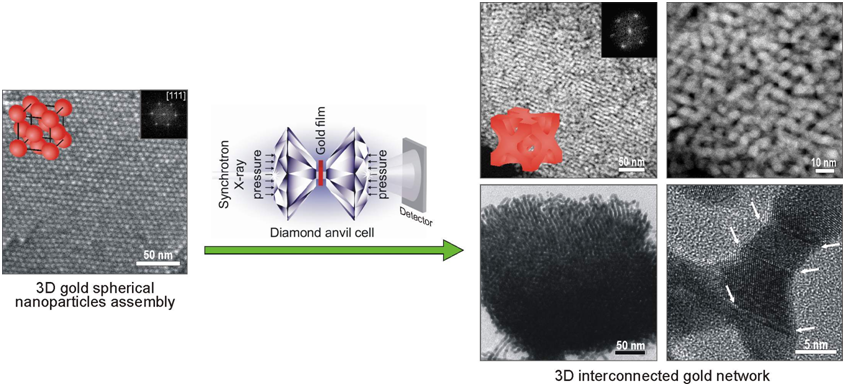
Recently a group led by Hongyu Fan (Sandia National Laboratory & the University of New Mexico) has developed a new method for forming gold networks. Their method is described in a recent paper by Huimeng Wu et al., published in the Journal of the American Chemical Society. Rather than starting with a solid alloy of gold and etching away parts of it, this method begins with unsupported gold nanoparticles only 5 nanometers across. The nanoparticles are loaded into a diamond anvil cell and then compressed at pressures up to 12 gigapascals (100,000 times higher than atmospheric pressure) to form porous gold networks.
The Cornell High Energy Synchrotron Source was used to make in situ small-angle x-ray scattering measurements which tracked the structure of the nanoparticles as the pressure was increased. For pressures below 9 gigapascals, the nanoparticles rearrange into a simple lattice, and return to their original state when the pressure is removed. However, at pressures above 9 gigapascals, the nanoparticles were irreversibly fused together and formed a three-dimensional network. These structures are mechanically stable and decompress slightly when returned to ambient pressure, but otherwise maintain their shape.
The resulting gold networks have features as small as 6 nanometers, and may provide a larger surface area per weight than supported nanoparticles. While this demonstration was done on a small scale in a diamond anvil press, the pressures required are readily accessibly by large-volume commercial presses [5], making it possible for these networks to be produced in bulk. It also does not require harsh chemical etchants as in other methods. With these advantages, fused gold nanoparticle catalysts could eventually displace supported nanoparticle catalysts in consumer products. While they might eventually become part of hydrogen fuel cells or micron-scale sensors [3], they could also help with other less glamorous problems – supported gold nanoparticle catalysts were first commercialized in bathroom air fresheners [1].
More information is available at the Fan group's web site at:
http://www.unm.edu/~hyfan
References:
[1] Hutchings, G.J. & Haruta, M.; A Golden Age of Catalysis: A perspective. Applied Catalysis A: General 291, 2-5 (2005)
[2] Haruta, M., Kobayashi, T., Sano, H. & Yamada, N.; Novel
Gold Catalysts for the Oxidation of Carbon Monoxide at a Temperature
Far Below 0.DEG.C. Chem. Lett. 405-408 (1987).doi:10.1246/cl.1987.405
[3] Corti, C.W., Holliday, R.J. & Thompson, D.T.; Commercial
Aspects of Gold Catalysis. Applied Catalysis A: General 291, 253-261 (2005)
[4] Erlebacher, J., Aziz, M.J., Karma, A., Dimitrov, N. & Sieradzki, K.; Evolution of
Nanoporosity in Dealloying. Nature 410, 450-453 (2001)
[5] Khvostantsev, L.G. & Slesarev, V.N.; Large-volume
High-pressure Devices for Physical Investigations. Phys.-Usp. 51, (2008)
* Alex Mellnik is a graduate student at the Cornell Physics Department.
