X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18
Bacteriophages are viruses which infect bacteria. Many of them consist of a rigid icosahedral capsid containing the phage's genetic material attached to a tail which penetrates the bacterial wall during the infection process. A complex structure known as the "portal" connects the tail and the capsid, and is required both to load the capsid with DNA (or RNA) and to mediate its injection into the host bacterium.
P22, which belongs to the Podoviridae family of short-tailed phages, has a large portal vertex structure consisting of 51 polypeptide chains of 5 different types. The Cingolani group (Thomas Jefferson University) has now determined a 3.25 Å crystal structure of a complex of the core domain of the P22 portal protein gp1 with multiple copies of the tail accessory factor gp4 (another component of the portal vertex), as well as a low resolution structure of intact gp1. This was a difficult project, due to the limited resolution of the data and the large size of the structures to be determined (18,500 residues in the asymmetric unit for the gp1-gp4 complex). Success was achieved using a model from electron microscopy as a starting point, followed by phase extension to the limit of the X-ray data. The protein used contained selenomethionine rather than normal methionine, and the positions of the large peaks due to selenium atoms provided assurance that the protein sequence was correctly placed in the electron density
In combination with previously determined X-ray and electron microscopy structures, the new work has enabled construction of a detailed model of the dodecameric portal of P22. The research is reported in Nature Structural & Molecular Biology:
A.S. Olia, P.E. Prevelige Jr., and G. Cingolani; "Three-dimensional Structure of a Viral Genome-delivery Portal
Vertex",
Nature Struct. 7 Mol. Biol.18, 597-603 (2011)
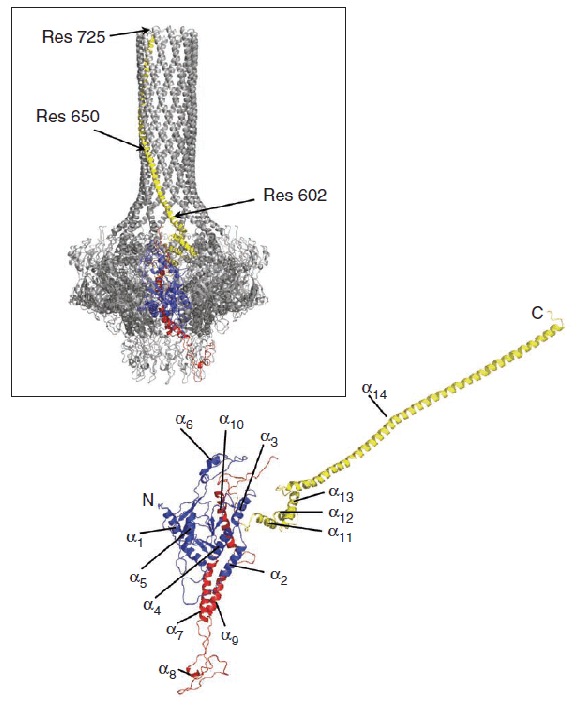
Structure of the gp1 portal protein monomer of P22. The red and blue regions are similar to those found in the related Phi29 and SPP1 phages, while the yellow barrel domain is absent or much shorter in the other phages. The inset shows how the monomer packs with another 11 copies to make up the portal dodecamer.
The P22 portal protein has 12-fold rotational symmetry, and consists of a funnel-shaped core connected to a long, mostly helical, tube resembling a rifle barrel. The core is similar to that found in other phages, such as SPP1 and Phi29, but the barrel is the first such structure seen in a portal protein. Double-stranded DNA would slide comfortably inside the barrel. Comparison of the gp1 core/gp4 and full-length gp1 structures indicates that binding of gp4 does not affect the structure of gp1, and the structures determined are consistent with EM structures, indicating their physiological relevance.
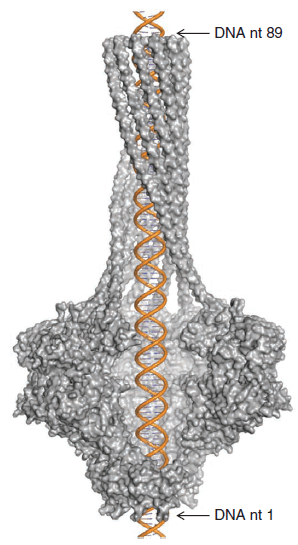
Model of double-stranded DNA inside P22 portal protein, with four protein monomers removed to show the interior of the channel.
In the intact virion, the barrel domain projects into the interior of the capsid. The authors propose that it serves a dual function: (1) during packaging of DNA, the end of the barrel can sweep out a circular path to neatly coil DNA along the interior wall of the capsid; (2) during injection of DNA into a bacterium, the long barrel domain stabilizes the linear momentum of the DNA as it is propelled along by the internal pressure in the capsid. Just as a rifle is more accurate and powerful than a pistol, DNA ejection through a long tube is more effective than through a short tube. Some phages have a long tail structure that serves this purpose, while P22 and similar phages have a short tail but a long internal barrel to achieve the same result.
Submitted by: Marian Szebenyi, MacCHESS, Cornell University
