X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18
Crystals consist of atoms arranged into periodic arrays. There has been much recent excitement about the use of nanometer-sized crystals (NC) for a wide variety of applications ranging from optoelectronics to catalysis. Recent interest has centered on whether NCs of a uniform size can themselves be assembled into a periodic array, thereby creating a crystal of nanocrystals (see figure). These nanocrystal superlattice arrays would have interesting new optoelectronic properties that do not exist with individual NC particles. Thus, it is crucial to understand and ultimately control the mechanisms governing NC superlattice formation, in order to realize such optoelectronic applications. Scientists at Cornell University have now developed a novel way to controllably create nanocrystal superlattices.
NCs can be formed of metals, insulators, or semiconductors such as lead sulfide (PbS) used in this study. Often these NC cores have beautiful shapes, such as the cuboctahedra – cubes with truncated corners – featured by PbS nanocrystals. Alkyl–chain based molecules (ligands, in the jargon for the field), in this case oleic acid -- the basic ingredient of olive oil -- cover surfaces of the NCs to prevent clumping and thus provide stability of the NC suspension in solutions. Upon casting from solution, NCs can pack into highly ordered superlattices.
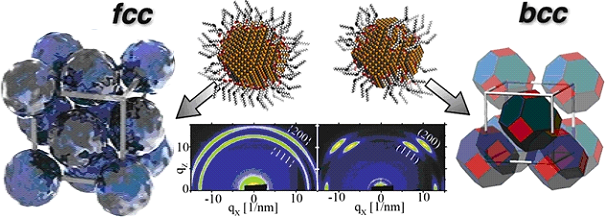
Figure:
In-situ grazing-incidence wide-angle scattering revealing random orientation of PbS nanocrystals in FCC superlattices and orientational order of nanocrystal facets in BCC superlattices. Whether FCC or BCC lattices are formed is a function of the ligand coverage.
Joshua Choi, a graduate student from the Cornell University Department of Applied and Engineering Physics and his collaborators found that the assembly of colloidal NCs into superstructures with long-range translational and orientational order is sensitive to the molecular interactions between ligands bound to the NC surface. They illustrated how ligand coverage on colloidal PbS nanocrystals can be exploited as a tunable parameter to direct the self-assembly of superlattices with predefined symmetry. Grazing-Incidence Small-Angle X-ray Scattering (GISAXS) showed that PbS NCs with dense ligand coverage assemble into face-centered cubic (FCC) superlattices, whereas NCs with reduced ligand coverage assemble into body-centered cubic (BCC) superlattices.
In order to probe the orientational ordering of NCs in their lattice sites, the researchers measured grazing-incidence wide-angle X-ray scattering (GIWAXS) patterns and found that NCs with dense ligand shells in FCC superlattices do not show orientational ordering; in contrast NCs with reduced ligand coverage showed high degree of orientational ordering. Surface chemistry characterization combined with density functional theory calculations suggested that the loss of ligands occurred preferentially on {100} facets (the cube faces) than on reconstructed {111} facets of the truncated corners of the cuboctahedra.
These results suggested that dense and isotropic ligand coverage around NCs render the interaction between NCs to be well approximated by spherical potentials, whereas anisotropic ligand distribution across different NC facets amplifies the role of non-spherical NC shape in the assembly and leads to the formation of superlattices with translational and orientational order. For this work Joshua Choi was awarded a MRS Poster Award at the 2010 Materials Research Society Meeting in Boston (photo, below). A paper on this work recently appeared in the Journal of the American Chemical Society [1] .

The work is an example of the excellent cross-disciplinary collaboration at Cornell. Choi is from the Department of Applied and Engineering Physics. Nanoparticle synthesis was performed in Tobias Hanrath’s lab in the department of Chemical and Biological Engineering. Hanrath is the principal investigator of this project and Choi’s thesis supervisor. Infra-red spectroscopy and transmission electron microscopy were performed using equipment at the Cornell Center for Materials Research. The structure determination of the highly ordered nanocrystal superlattices was performed at the Cornell High Energy Synchrotron Source (CHESS) D1 station using a combination of Grazing Incidence Small and Wide Angle Scattering apparatus assembled by CHESS staff scientist Detlef Smilgies. The NC surface chemistry was probed with XPS in Jim Engstrom’s group in Chemical and Biological Engineering. Richard Hennig’s group (Materials Science and Engineering) performed quantum chemical calculations of the ligand binding energies on the different NC facets
Reference:
1) Joshua J. Choi, Clive R. Bealing, Kaifu Bian, Kevin J. Hughes, Wenyu Zhang, Detlef-M. Smilgies, Richard G. Hennig, James R. Engstrom, and Tobias Hanrath,
“Controlling Nanocrystal Superlattice Symmetry and Shape-Anisotropic Interactions through Variable Ligand Surface Coverage”, J. Am. Chem. Soc.
133 pp. 3131-3138 (2011)
(http://pubs.acs.org/doi/abs/10.1021/ja110454b)
Submitted by: Arthur Woll, CHESS, Cornell University
