X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18
A major challenge for developing next-generation fuel cells, which generate electricity by combining hydrogen and oxygen, is developing catalysts that are cheaper and longer-lasting alternatives to those based on platinum. A search for alternative catalyst materials among binary and ternary compounds is underway, but the variety of these compounds is virtually infinite. To make the problem more tractable, researchers at the Energy Materials Center at Cornell (EMC2) have developed a novel technique to screen thousands of potential catalyst materials in parallel.
The approach begins with fabrication of a “composition spread” thin film, on which the composition ratios of different elements varies continuously across a substrate, typically a 4” silicon wafer. Next, spectroscopic techniques are used to identify regions of the film with strong catalytic activity. Finally, the composition and structure of those regions must be identified. For this final step, EMC2 has joined forces with scientists at the Cornell High Energy Synchrotron Source (CHESS) to quickly and efficiently characterize the composition spread thin films.
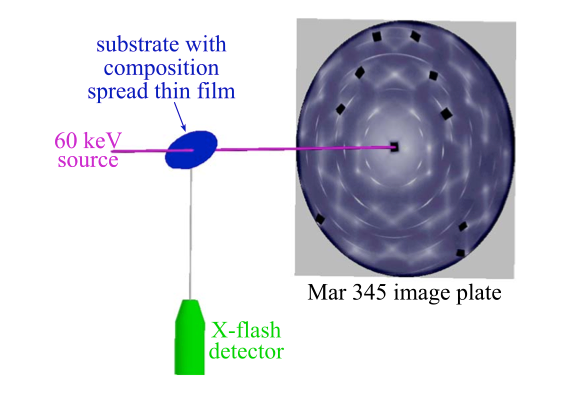
From reference 1.
The high-throughput technique combines high-energy diffraction with x-ray fluorescence spectroscopy to obtain the structure, texture, composition, and thickness for each area of the film. Details of the approach are described in Gregoire et.al.1, using a binary composition spread of PtxRu1-x. The researchers have developed algorithms to process datasets which include complex features unique to thin films and alloys2. The group has applied the technique to a variety of intermetallic systems. In the case of PtxTa1-x, they observed a substantial improvement in catalytic activity for methanol oxidation, which they attributed to interactions at the surface between Pt and partially-reduced TaOy3. The group has also applied the technique to a variety precious-metal/transition-metal carbide systems4, yielding surprising results with important implications for fuel cell fabrication. In particular, the solubility of precious metals in a carbon deficient tungsten carbide phase has important implications for fuel cell electrode fabrication and suggests that investigation of new ternary carbide alloys as fuel cell catalysts are a fruitful direction for future research.
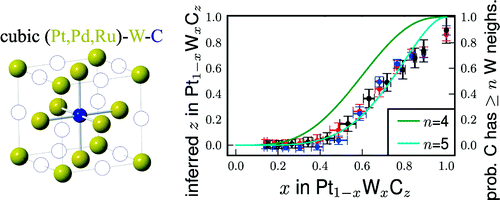
From reference 2.
References:
1) J.M. Gregoire, D. Dale, A. Kazimirov, F.J. DiSalvo, and R.B. van Dover,
"High Energy X-ray Diffraction/x-ray Fluorescence Spectroscopy for
High-throughput Analysis of Composition Spread Thin Films," Review of Scientific Instruments
80 123905 (2009) (http://rsi.aip.org/resource/1/rsinak/v80/i12/p123905_s1)
2) John M. Gregoire, Darren Dale, and R. Bruce van Dover, “A Wavelet Transform Algorithm for Peak Detection and Application to Powder X-ray Diffraction Data,” Review of Scientific Instruments 82, 015105 (2011) (http://rsi.aip.org/resource/1/rsinak/v82/i1/p015105_s1)
3) John M. Gregoire, Michele E. Tague, Sophie Cahen, Sahr Khan, Héctor D. Abruña, Francis J. DiSalvo, R. Bruce van Dover,
“Improved Fuel Cell Oxidation Catalysis in Pt1−xTax,” Chemistry of Materials
2010 22 (3), 1080-1087 (2010)
(http://pubs.acs.org/doi/abs/10.1021/cm9020782)
4) J.M. Gregoire, M.E. Tague, E.H. Smith, D. Dale, F.J. DiSalvo, H.D. Abruña, R. G. Hennig, and R. B. van Dover,
"Phase Behavior of Pseudobinary Precious Metal-Carbide Systems," Journal of Physical Chemistry C
114 (49), 21664-21671 (2010)
(http://pubs.acs.org/doi/abs/10.1021/jp1092465)
Submitted by: Arthur Woll and Darren Dale, CHESS, Cornell University
