X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18
HIV reverse transcriptase (RT) is a key protein that allows the human immunodeficiency virus to multiply inside infected cells. As such, RT is a major target of drug design efforts. Because of the naturally rapid mutation of HIV, no single drug is completely effective and existing treatments often consist of cocktails of multiple drugs.
Fourteen of the twenty-six FDA approved anti-retroviral AIDS drugs specifically inhibit the action of RT by mimicking naturally occurring nucleotides required by the protein. (http://www.fda.gov/ForConsumers/byAudience/ForPatientAdvocates/HIVandAIDSActivities/ucm118915.htm). A second class of important drugs is the so-called the non-nucleoside reverse transcriptase inhibitors (NNRTIs). Exactly how NNRTIs interrupt the function of RT is not well understood; a variety of mechanisms have been proposed based on various biophysical measurements. Now researchers from the Center for Advanced Biotechnology of Rutgers University have used the Cornell High Energy Synchrotron Source to solve a series of three key complexes of RT: (Kalyan Das, Sergio E Martinez, Joseph D Bauman, and Eddy Arnold; "HIV-1 Reverse Transcriptase Complex with DNA and Nevirapine Reveals Non-nucleoside Inhibition Mechanism", Nature Structural & Molecular Biology 19, (2012) pp. 253-259). (Link to NSMB article - may need to sign in)
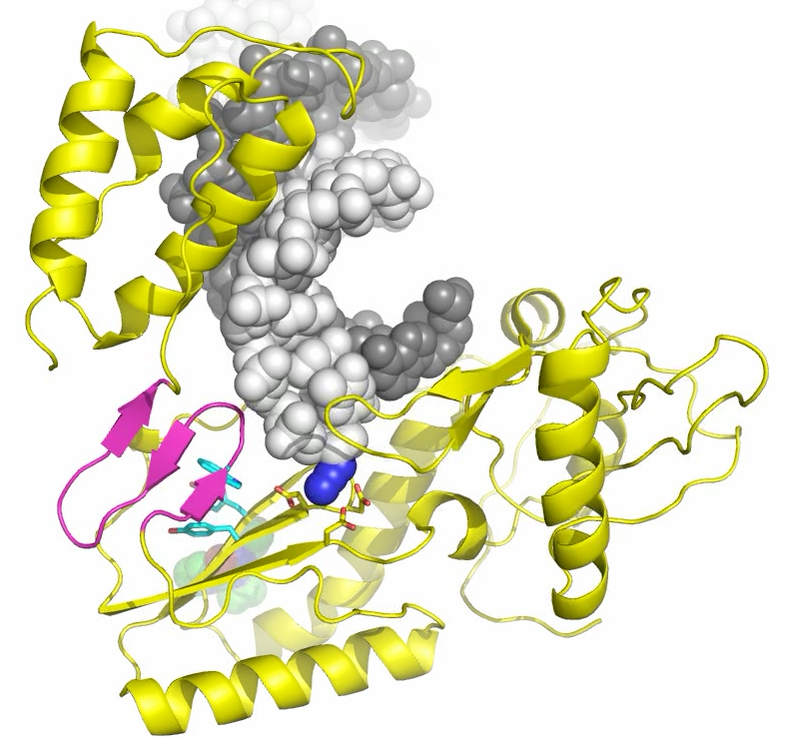
Still image taken from movie generated for article
"HIV-1 Reverse Transcriptase Complex with DNA and Nevirapine Reveals Non-nucleoside Inhibition Mechanism", Nature Structural & Molecular Biology 19, (2012) pp. 253-259 with permissions.
The presence of DNA is essential for understanding RT function, so Das et al. chemically cross-linked DNA to RT in a way that trapped the RT structure in the physiologically important polymerase-competent form. Once crystals of this binary DNA-RT complex were obtained, the researchers were able to soak in two different drug molecules: AZTTP (a classic nucleoside inhibitor), and nevirapine (an important non-nucleoside inhibitor).
A critical innovation in these studies was selection of a crystal form of the binary DNA-RT complex that contained enough open space (>50% water) for the protein RT to be able to change its shape naturally in response to drug binding. The differences between AZTTP and nevirapine complexes became clear: Like a hand with fingers, RT closes in response to AZTTP, but results in little change in the region of bound DNA. Nevirapine, on the other hand, causes a number of significant changes in how DNA is held by RT; DNA shifts from its natural binding pocket disrupting key catalytic contacts.
These insights into how non-nucleoside drugs disrupt the conformation of HIV reverse transcriptase will not only support new AIDS drug development efforts, but may also help in the effort to develop allosteric inhibitors for other disease targets such as hepatitis C NS5B polymerase and bacterial RNA polymerase. Previous work from CHESS by the Arnold lab at Rutgers enabled the design of two of the FDA-approved treatments for HIV infection: Edurant/rilpivirine/TMC278 and Intelence/etravirine/TMC125, both of which are marketed by Johnson & Johnson.
Submitted by: Richard Gillilan, MacCHESS, Cornell University
06/22/2012
