X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18
Assembly of nanoparticles into ordered superlattices opens up many potential applications such as sensors, catalysts, and novel optical materials. Nanoparticles consist of an inorganic core that can be a metal, a semiconductor or an insulator, and an organic ligand corona that makes particles soluble and prevents them from sticking together irreversibly. Another distinct feature is the shape of the core – recently spheres, rods, cubes, octahedra and many others have been synthesized. While spheres readily self-assemble into close-packed structures, recently it has been found that also non-spherical particles can form highly ordered lattices – sometimes with unusual properties! For instance James Fang’s group at SUNY Binghamton has recently demonstrated how to synthesize monodisperse nanometer-scale octahedra, in short nanoctahedra, from platinum based alloys, such as Pt3Ni and Pt3Cu2. When these octahedra crystallize, they form a very open BCC structure with the nanoctahedra tips touching each other. In such a structure the inorganic cores fill only 33% of the unit cell volume. Due to the sharp corners and tips the ligand sphere is quite anisotropic – on the facets the ligands, a mix of oleic acid and oleylamine, can pack closely, while at corners and tips they are highly frustrated. This anisotropy appears to stabilize the open structure.
While the assembly of spherical colloids into close-packed FCC superlattices is well understood as an entropic process in terms of the Kirkwood-Alder mechanism, much less is known about the crystallization of nanoparticles. In nanoparticles the ligand length of around 2 nm is on the same order of magnitude as the core diameters of typically 2-10 nm. Hence the ligands play a much more significant role in nanoparticle crystallization than in the larger colloids with core diameters of 100 nm to several microns. For soft spherical particles such as block copolymer micelles a soft Kirkwood-Alder transition has been identified. The outer ligand shell facilitates the formation of either FCC structures that minimize enthalpy, or BCC structures which maximize entropy. The question arises whether this is still the case when the ligand shell becomes anisotropic.
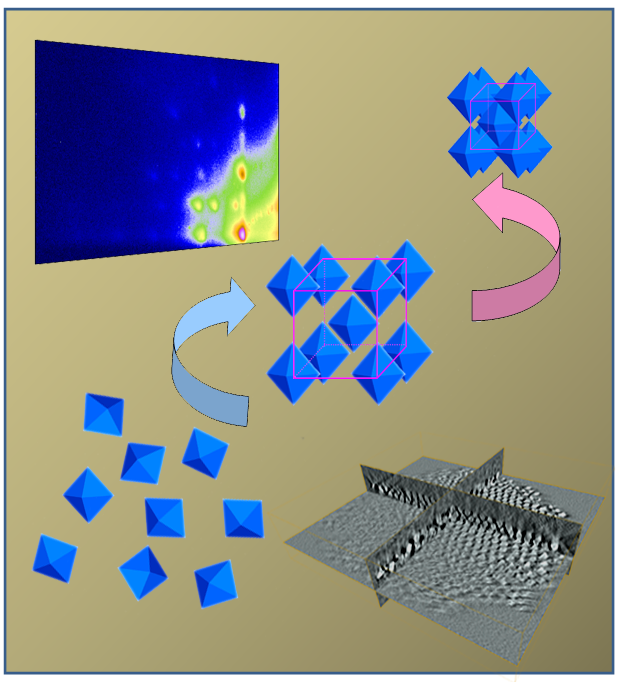
Kirkwood-Alder transition in the soft crystallization of Pt3Cu2 nanoscale octahedra. At first particles assemble from solution to an open bcc structure with still a lot of solvent in the lattice as followed using in-situ GISAXS measurements (top left). In the dried state a very open structure with tip-to-tip oriented octahedral is formed, as characterized with electron tomography (bottom right).
In recent experiments a CHESS D1 station, the SUNY team and CHESS staff scientist Detlef Smilgies carefully studied the drying of a dropcast solution of Pt3Cu2 nanoctahedra suspended in hexane using the D1 controlled vapor sample environment and grazing-incidence small-angle scattering (GISAXS). The hexane vapor pressure in the cell was controlled by a reservoir of liquid hexane and a gentle helium purge gas flow. Under saturated hexane vapor the nanoparticles stayed in a liquid-like disordered phase. By slowly increasing the helium flow, the particles could be brought to crystallize, and upon reducing the flow again, to go back into the disordered phase. At the crystallization threshold the unit cell was significantly larger (a=16.7 nm) than in the completely dried phase (a=15.3 nm). During drying the unit cell size shrank continuously, as solvent was leaving the lattice. Moreover, the ratio of unit cell volumes at crystallization and in the dry state follows closely the prediction of Kirkwood-Alder theory. Hence it could be shown, that even highly anisotropic particles with a high degree of orientation on their lattice sites can be understood within the Kirkwood-Alder mechanism of soft crystallization. The tunability of the lattice spacing could be important for sensor and optical applications.
Reference:
J. Zhang, Z. Luo, B. Martens, Z. Quan, A. Kumbhar, N. Porter, Y. Wang, D. M. Smilgies, and J. Fang; "Reversible Kirkwood-Alder Transition Observed in Pt3Cu2 Nanoctahedron Assemblies under Controlled Solvent Annealing/Drying Conditions", Journal of the American Chemical Society 134 (34), 14043-14049 (2012)
Submitted by: Detlef Smilgies, CHESS, Cornell University
10/17/2012
