X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18
Legionella pneumophila is a bacterium which can be found in the stagnant water of poorly-maintained air conditioning systems and similar environments, and is linked to sporadic outbreaks of the human affliction known as Legionnaires' disease. When L. pneumophila cells are inhaled into the lungs, they are swallowed by immune cells known as macrophages. They may then secrete proteins into the macrophages which alter the vacuoles in which they are contained into a more hospitable environment for themselves.
Recent evidence indicates that the manipulation performed by L. pneumophila involves skewing the host's balance of phosphoinositides. These are lipid components of cellular membranes which have several sites for phosphorylation, and the presence or absence of phosphate at the various sites (3,4,5) can cause the cell to behave in different ways. Thus, phosphoinositides play an important role in membrane trafficking, the important process that directs specific membrane proteins to the specific parts of the cell where they are needed.

A team of researchers from Cornell University and Purdue University has identified SidF, one of the proteins secreted by L. pneumophila, as a phosphatidylinositol polyphosphate 3-phosphatase. Through a series of biochemical assays, they identified the specific reaction carried out by SidF as the removal of the 3-phosphate group from PI(3,4)P2 and PI(3,4,5)P3. By deleting the SidF gene from the bacterium, they verified that SidF is necessary for manipulation of the vacuole membrane by creating extra PI(4)P, through which proteins bind to the membrane.
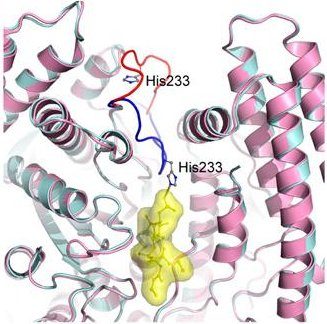
Then the researchers crystallized the SidF protein and solved its molecular structure through X-ray crystallography with data collected at CHESS. They solved the structure of the enzyme alone, and the enzyme with a phosphoinositide bound in the active site, revealing considerable details of the binding mechanism. This latter structure utilized an inactive C645S mutant of SidF to keep it from turning over the substrate, PI(3,4)P2. This work greatly enlarges our understanding of the infectivity of L. Pneumophila at both the biochemical and molecular levels.
The lead author of the resulting paper is FoSheng Hsu, Cornell graduate student and award-winning interpretive dancer. Other authors from Cornell include Hsu's advisor, assistant professor Yuxin Mao, and graduate student Lucy Brennan. Participants from Purdue include associate professor Zhao-Qing Luo, graduate student Wenhan Zhu and visiting student Lili Tao.
FoSheng Hsu, Wenhan Zhu, Lucy Brennan, Lili Tao, Zhao-Qing Luo, and Yuxin Mao; "Structural Basis for Substrate Recognition by a Unique Legionella Phosphoinositide Phosphatase", PNAS vol.109, no. 34, pp. 13567-13572; doi: 10.1073/pnas.1207903109 (August 21, 2012)
Protein Data Bank (PDB) entries 4FYE, 4FYF, and 4FYG.
Submitted by: David J. Schuller, MacCHESS, Cornell University
10/11/2012
