X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18
The largest scale application of homogeneous catalysis (by dollar amount of product sold) is hydrosilylation, a process used to manufacture chemicals used in consumer products from iPhones to beer. On the industrial scale, the catalyst used is platinum-based, but the reaction can now be done using a cheaper, iron-based system in which no disadvantageous side products are formed1.
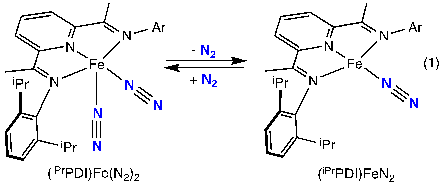
The bis(imino)pyridine iron pre-catalyst used in these reactions is in equilibrium between four- and five-coordinate bound compounds in solution (illustrated below), but little is known about the the effect of N2 lability, if any, upon the electronic structure of this catalytic precursor.
New spectroscopic tools are needed to understand changes in electronic structure at the metal center in this and many more complex processes. Chantal Stieber and colleagues2 combined X-ray Emission (XES) and Absorption (XAS) spectroscopies for this purpose. The methods are complimentary probes of the electronic states directly involved in chemistry. XES senses the most weakly bound electrons, while XAS reports on the lowest unfilled levels. Both methods are element-specific and can utilize the same setup as illustrated in the figure below.
In the present study, the electronic structures of four- and five-coordinate bis(imino)pyridine iron dinitrogen complexes were examined. The work shows that dissociation of a dinitrogen ligand results in measureable differences in the ligand field at the iron center. From XAS we learn that the iron oxidation state is unchanged, and transitions to ligand π* orbitals can be observed. Although XES can distinguish between 1 or 2 bound dinitrogen molecules, it cannot distinguish between the two bis(imino)pyridine iron oxidation states or electronic structures. With these analytical tools in place, progress can be made in characterizing and further developing effective alternatives to platinum-based catalysts.
The lead author, Chantal Stieber, is a graduate student in the Chirik group at Princeton University and the DeBeer group at Cornell & Max-Planck-Institut für Bioanorganische Chemie. The X-ray Emission work was carried out in collaboration with CHESS scientist Ken Finkelstein at the CHESS C1 station.
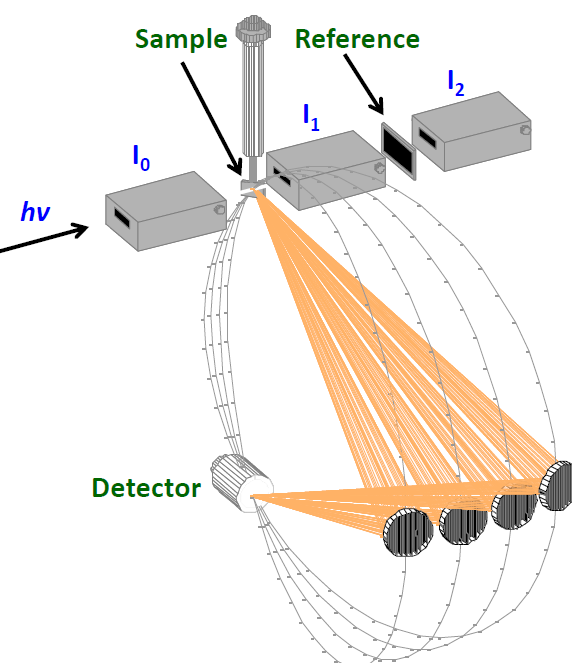
The experimental layout in the C1 hutch is shown in the figure. X-rays incident from the left pass through ion chambers (I0, I1, I2) to measure the intensity of the x-ray beam at various points. X-ray emission from the sample is reflected from curved monochromator disks fabricated from semiconductor wafers onto the x-ray detector. (Figure courtesy of Mario Delgado-Jaime)
[1] A.M. Tondreau, C.C.H. Atienza, K.J. Weller, S.A. Nye, K.M. Lewis, J.G.P. Delis, P.J. Chirik; Science 335, 567-570 (2012)
[2] S.C.E. Stieber, C. Milsmann, J.M. Hoyt, Z.R. Turner, K.D. Finkelstein, K. Wieghardt, S. DeBeer, P.J. Chirik; Inorg. Chem. 51, 3770-3785 (2012)
Submitted by: Ken Finkelstein, CHESS, Cornell University
04/24/2012
