X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18
Cells synthesize proteins by transcribing genetic information from DNA onto messenger RNA (mRNA) molecules. The mRNA conveys the instructions for protein synthesis to ribosomes, which use this information to make the proteins. Various helper proteins are involved in this transcription process. The Gehring group and co-workers (McGill and Concordia Universities, Montreal, Canada) report in the journal Molecular Cell how they used X-ray methods to elucidate how this transcription process works.
Allosteric (i.e., shape changing) interactions between domains of the RNA binding protein PABP regulate its binding to another protein to form the closed-loop structure of actively transcribed mRNAs. The process of protein synthesis requires the proper assembly of a protein•mRNA complex that links the 5' and 3' ends of the mRNA. This complex is important for the translational regulation of gene expression and efficient translation of the mRNA into protein (Wells et al., 1998; Amrani et al., 2008). Poly(A)-binding protein (PABP) plays a central role in the complex through its interactions with the 3’ poly(A) tail of mRNA and the protein eukaryotic initiation factor 4G (eIF4G). Together the two proteins, PABP and eIF4G, link the two ends of the mRNA to form the mRNA closed-loop. This brings together the regulatory elements in the 5' and 3' ends of the mRNA and increases the rate of translation.
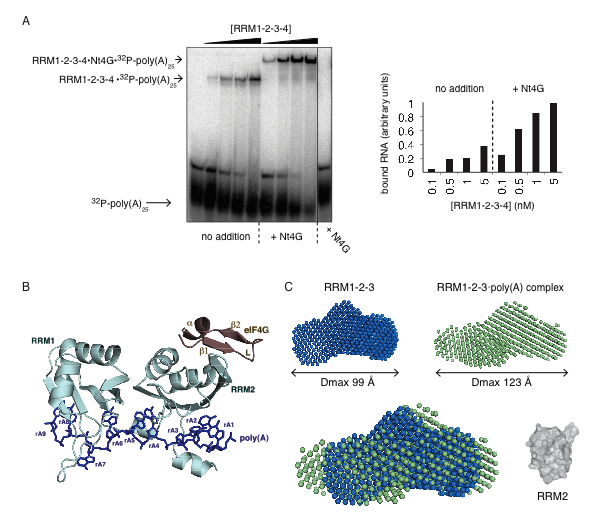
Figure 1. Cooperativity of poly(A)•PABP•eIF4G complex formation.
(A) Gel shift assay of 32P-labeled poly(A)25 RNA binding to PABP domains RRM1 2 3 4 in the absence and presence of an N-terminal fragment of eIF4G (Nt4G). Quantification (right panel) confirms the higher affinity of PABP for RNA in the presence of Nt4G.
(B) X-ray crystal structure of the ternary complex of eIF4G (178-203) (brown) bound to PABP RRM1 2 (light blue) in the presence of poly(A)11 (dark blue). The eIF4G peptide binds to the a-helical surface of RRM2 while the poly(A) RNA binds to the b-sheet surface of both RRMs.
(C) The ab initio SAXS models of the free RRM1 2 3 fragment (blue) and RRM1 2 3•poly(A)11 complex (green) with their corresponding maximum diameters (Dmax). The overlay of the two shapes shows the change in the conformation upon poly(A) binding with the crystal structure of the isolated RRM2 domain (grey) shown for comparison.
By combining X-ray crystallography, small-angle-X-ray scattering (SAXS) collected at CHESS and the Canadian Light Source with NMR data, the Gehring group studied the mechanisms that promote assembly of the poly(A)•PABP•eIF4G (Figure 1; Safaee et al., 2012). Gel electromobility shift assays and isothermal titration calorimetry experiments showed that the complex assembles in a cooperative manner arising from interdomain allostery between the first two RNA-binding domains of PABP. The allosteric interactions decrease the affinity of PABP for RNA in the absence of eIF4G and increase the affinity in its presence. Small angle X-ray scattering showed that complex formation leads to a compact structure, which was crystallized to reveal the atomic interactions responsible for the specificity of the molecular interactions. The crystal structure explains the previous observations of the enhancement translation initiation by polyA RNA in trans (Borman, 2002) and serves as a model for interdomain allostery in RNA binding proteins. The resulting insights as to the structural steps involved in this complex process help scientists better understand the protein factory on which we are all dependent.
References:
Amrani, N., Ghosh, S., Mangus, D.A., and Jacobson, A. (2008). Translation factors promote the formation of two states of the closed-loop mRNP. Nature 453, 1276–1280.
Borman, A.M. (2002). Free poly(A) stimulates capped mRNA translation in vitro through the eIF4G-poly(A)-binding Protein interaction. J Biol Chem 277, 36818–36824.
Safaee, N., Kozlov, G., Noronha, A.M., Xie, J., Wilds, C.J., Gehring, K. (2012). Interdomain allostery promotes assembly of the poly(A) mRNA complex with PABP and eIF4G. Mol Cell. 48, 375-386.
Wells, S.E., Hillner, P.E., Vale, R.D., and Sachs, A.B. (1998). Circularization of mRNA by eukaryotic translation initiation factors. Mol. Cell 2, 135–140.
Submitted by: Chae Un Kim, MacCHESS, Cornell University
3/28/2013
