X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18
Highly organized fusion of nanoparticles upon heating
Ostwald ripening is a familiar effect – think of small water droplet condensing on a cold window pane. Sooner or later larger droplets form that will grow at the expanse of the smaller ones. Now imagine this random process proceeding in a highly organized way and with formation of symmetric patterns – such a behavior would seem rather unusual.
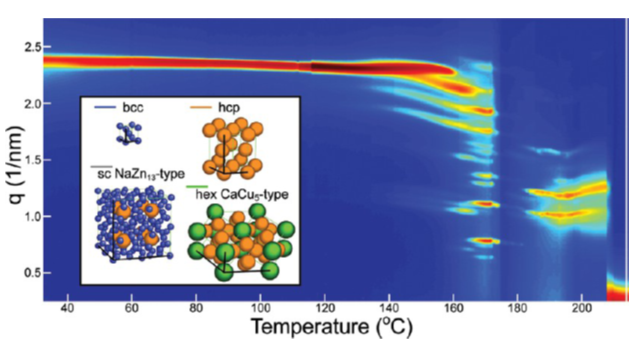
Figure 1: Evolution of integrated small-angle scattering patterns as a function of sample temperature. Four ordered phases are observed: body-centered cubic at room temperature, hexagonal close-packed above 140°C, single cubic AB13 above 160°C and hexagonal AB5 above 180°C. The latter three ordered phases are binary superlattices formed by larger fused gold nanocrystals and the original small particles.
A research team from the Korgel group at the University of Texas at Austin (UTA) found that small gold nanoparticles do exactly that, even in three dimensions [1]. Brian Goodfellow and his fellow students observed that such tiny particles of a size smaller than 2 nm form a body-centered cubic lattice when dropcast on a silicon wafer or a thin glass slide. After heating the deposits to above 140°C, new diffraction features appear, indicating a new hexagonal crystal phase has formed. A similar change in the scattering pattern occurs at 160°C yielding a single cubic AB13 structure and at 180°C yielding a hexagonal AB5 phase. In conjunction with transmission electron microscopy, it was found that the new phases consist of regular arrangements of smaller and larger gold nanoparticles. Such phases have previously been observed in the controlled assembly of binary nanoparticle super lattices [2].
In the case of the gold particles, heating seems to cause some kind of self-organized ripening with particles only fusing at certain positions in the original superlattice, and then forming a binary superlattice of larger fused gold nanocrystals and the original small gold particles. The final phase formed at over 220°C was a random network of fused gold particles – at this point the lattice symmetry was finally lost.
Says CHESS D1 staff scientist Detlef Smilgies: “This is the most amazing thing I have seen to date on the behavior of nanocrystal superlattices! Usually, if you mess with a superlattice, it just falls apart and becomes amorphous. But these gold particles just kept producing richer and richer scattering patterns.”
Particles were studied on heating stages mounted to the D1 goniometer. For transmission small-angle scattering (SAXS), samples were dropcast on a thin glass slide and covered by another thin glass slides on a Mettler heating stage provided by the Ober group at Cornell. For grazing-incidence SAXS measurements particles were dropcast on a silicon wafer and heated on a custom stage supplied by the UTA group. GISAXS revealed that the BCC, HCP, and HEX AB5 phases had a strong preferential orientation with regard to the substrate which indicates that they nucleated at the interface.
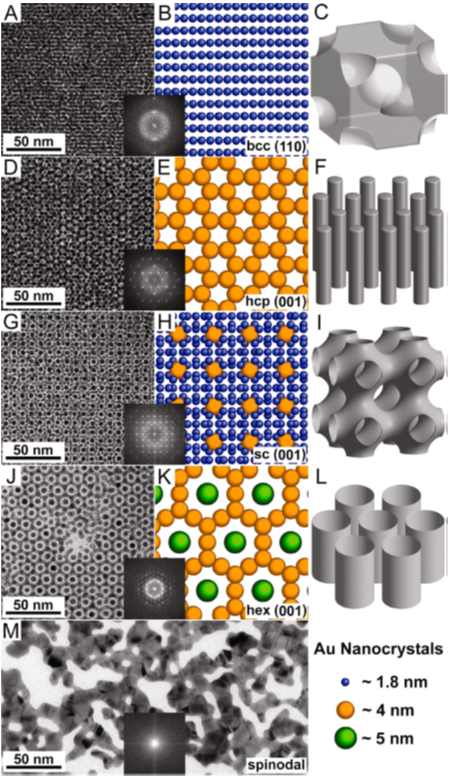
Figure 2: Analogy of observed binary superlattices with block copolymer phases (right column). The final structure with a complete loss of ligands was a random network of fused gold nanocrystals.
The initial step in the transformation seems to be driven by ligand loss at elevated temperature which facilitates the fusion of adjacent particles. Moreover, the small initial particles have a much reduced melting point. The UTA team explained the surprising self-organization in an analogy to block copolymer phases [3]: the original BCC phase bears a strong resemblance to BCC spheres, the HCP and HEX AB5 phases to the hexagonal cylindrical phase and the cubic AB13 phase to the plummer’s nightmare bicontinuous phase. Apparently the free energy of the interacting ligand systems of the particles is minimized by assuming such phases known from block copolymers and surfactants. The self-organized fusion of gold particles provides a promising route for preparing binary superlattices from a simple monodisperse precursor.
References:
[1] Brian W. Goodfellow, Michael R. Rasch, Colin M. Hessel, Reken N. Patel, Detlef-M. Smilgies, and Brian A. Korgel: “Ordered Structure Rearrangements In Heated Gold Nanocrystal
Superlattices”, Nano Lett. (accepted in October 2013).
[2] Elena V. Shevchenko, Dmitri V. Talapin, Nicholas A. Kotov, Stephen O’Brien, and Christopher B. Murray: “Structural diversity in binary nanoparticle superlattices”,
Nature 439, 55-59 (2006).
[3] Brian W. Goodfellow and Brian A. Korgel: “Perspective: Reversible Solvent Vapor-Mediated Phase Changes in Nanocrystal Superlattices”, ACS Nano 5, 2419–2424 (2011).
Submitted by: Detlef Smilgies, CHESS, Cornell University
11/1/2013
