X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18
Unlike humans, plants lack the immune system that’s required to fend off attacks from pathogens. Plants rely on a nonspecific response to deal with an outside attack (in humans, this type of response is known as innate immunity). Examples of these plant defense mechanisms include the production of pathogen-degrading enzymes, the production of chemicals (sometimes toxic), and deliberate cell suicide. In a recent study by the group of structural biology professor Michael Malkowski of SUNY Buffalo, new light was shed on the reaction mechanism and substrate specificity of an enzyme that belongs to the pathogen inducible oxygenase family [1]. As part of the host defense response in Arabidopsis thaliana, levels of the heme-containaing α-Dioxygenase (Ath α-DOX) are up-regulated to produce oxygenated fatty acids, chemicals which are often toxic to pathogens. Malkowski and coworkers report a high resolution crystal structure of Ath α-DOX, the first crystal structure of its kind (Figure 1).
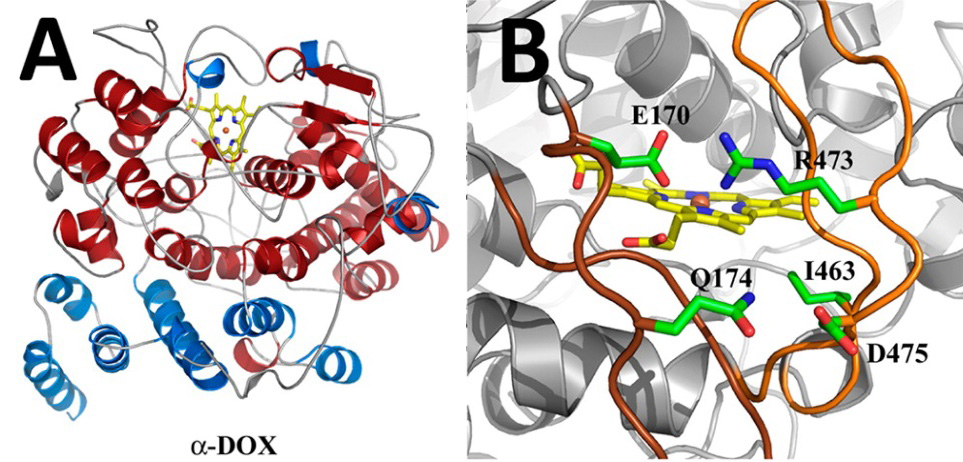
FIGURE 1. Near atomic resolution crystal structure of Ath α-DOX. A) Cartoon representation depicting the secondary structure elements found in Ath α-DOX. Elements that differ between COX and α-DOX are depicted in blue, and conserved regions between the two are depicted in red. B) Extended inserts of Ath α-DOX; view of the ionic interactions between the two inserts that serve to stabilize and rigidify the loops.
Albeit low in sequence identity, α-DOX is functionally related to cyclooxygenase (COX) and linoleate diol synthase (LDS), both of which can oxygenate fatty acids. A previous study, also authored by the Malkowski group, identified many of the key residues required for catalysis in the α-DOX enzymes [2]. In that study COX-1 from O.aries served as a structural surrogate for homology modeling. Although homology modeling can provide an accurate description of regions of high structural conservation, it often fails to answer questions regarding regions of low sequence identity. Coupled with mutagenesis work, the crystal structure of the mostly α-helical enzyme provides new insight into the molecular determinants of the α-DOX reaction, heme-iron stability and the binding modes for both substrate and the structural calcium ions (Figure 2).
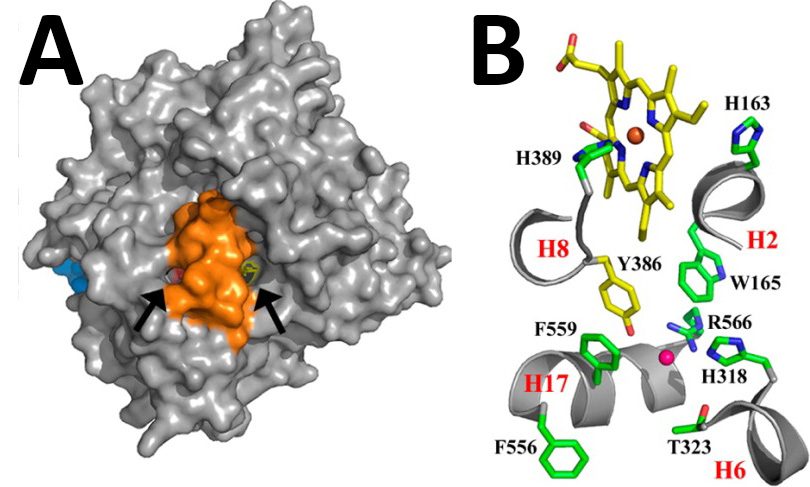
FIGURE 2. Substrate binding and active site. A) Surface representation of the Ath α-DOX showing the location of the two small channels (black arrows) that provide access to the heme. B) Close-up of the active site residues in Ath α-DOX.
The high-resolution data for apo wild-type Ath α-DOX were collected at the A1 beamline at CHESS. The findings of this research study were published in Biochemistry.
References:
- Goulah C. C., Zhu G., Koszelak-Rosenblum M., Malkowski MG. (2013) The crystal structure of α-Dioxygenase provides insight into diversity in the cyclooxygenase-peroxidase superfamily. Biochemistry. 52(8), 1364-72.
- Koszelak-Rosenblum M., Krol A. C., Simmons D. M., Goulah C. C., Wroblewski L., Malkowski M. G. (2008) His-311 and Arg-559 are key residues involved in fatty acid oxygenation in pathogen-inducible oxygenase. J Biol Chem. 283(36), 24962-71.
Submitted by: Tiit Lukk, MacCHESS, Cornell University
7/22/2013
