X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18
Facile synthesis of novel energy materials is necessary to tackle ever growing global energy demands. Cobalt oxide nanomaterials, in particular Co3O4 nanoparticles, are promising candidates for use in batteries, supercapacitors, and as electrocatalysts. The Robinson Group, in the Materials Science and Engineering department at Cornell University, has discovered important insights into methods to produce Co3O4 nanoparticles in a simple air oxidation process [1]. The nanoparticles have a high ratio of {110} surface planes making them ideal for use as catalysts. Insight into the formation mechanism for these particles will help enable advanced tailoring of composition and surface morphology for individual energy applications.
To create hollow Co3O4 nanoparticles, single-crystalline cobalt nanoparticles (Fig. 1a) are oxidized in air at 200°C, converting them to polycrystalline hollow CoO nanoparticles (Fig. 1b), and finally to polycrystalline hollow Co3O4 nanoparticles (Fig. 1c). Particle hollowing, observed during the air oxidation process, is due to the nanoscale Kirkendall effect which arises from a difference in diffusion rates between anions (O) and cations (Co).
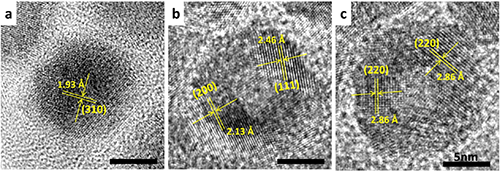
Figure 1. Transmission electron microscope images of: (a) single-crystalline cobalt nanoparticle starting material, (b) intermediate polycrystalline CoO nanoparticle during the air oxidation process, and (c) polycrystalline Co3O4 nanoparticle final product.
Information about both long- and short-range order in the nanoparticles throughout the oxidation process was measured using X-ray diffraction (Fig. 2a) and extended X-ray absorption fine structure (Fig. 2b), respectively. Interestingly, although the diffraction pattern of the starting material shows only a single-crystalline cobalt phase, extended X-ray absorption fine structure (EXAFS) reveals the presence of an amorphous CoO phase. The combination of X-ray diffraction and EXAFS allows for a much deeper understanding of the air oxidation process, allowing for identification of intermediate phases not “seen” by diffraction alone. These observations allowed the Robinson Group to produce an understanding of the air oxidation process not previously known. After 90 minutes of air oxidation the particles are completely converted into hollow polycrystalline Co3O4 (Fig. 2a and 2b). Attempts to obtain Co3O4 nanoparticles from the analogous solution-phase process did not produce Co3O4 even after reacting at 200°C for 3 hours, whereas the air oxidation goes completely too high quality Co3O4 in only 90 mins. The Co3O4 nanoparticles could then easily be dispersed in an organic solvent making them an ideal source of solution-processable colloidal, monodisperse Co3O4 nanoparticles for use in catalysis.
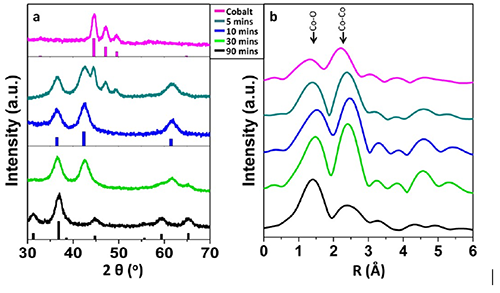
Figure 2. (a) X-ray diffraction and (b) extended X-ray absorption fine structure spectra measured on a representative set of ex situ samples from the cobalt nanoparticle air oxidation process.
Recently I caught up with Don-Hyung Ha, a graduate student working in the Robinson Group on synthesizing novel nanomaterials, and asked him why CHESS was so important for their research. Here is an excerpt of that conversation:
Q. Why is it so important to understand the air-oxidation process of Co nanoparticles (NPs)?
A. The air oxidation of Co NPs is a simple and robust method to synthesize cobalt oxide NPs. Moreover, we can tune the phase of cobalt oxides between CoO and Co3O4. To obtain a pure phase and optimize their properties, it is crucial to understand the oxidation process.
Also this NP oxidation reaction results in hollow structures due to the nanoscale Kirkendall effect, which arises from the difference in diffusion rates between the anion and cation. However the atomic structural evolutions and diffusion processes during NP oxidation is not well-understood.
Q. How did this research benefit from getting access to synchrotron beam-time at CHESS?
A. This study was based on extended X-ray absorption spectroscopy (XAS) measurements which require a high energy synchrotron source. Previous studies on this and similar systems didn't look at the short-range order through methods like XAS, and they weren't able to see the intricate mechanisms that we saw. XAS at CHESS was essential to fully resolve the atomic structural evolution during the oxidation.
Q. What was the most important observation your group made based on the x-ray absorption data collected at CHESS?
A. We extracted the atomic % of cobalt and oxygen atoms based on the structural contributions from the EXAFS fitting models for the samples. This provides the kinetics of the oxidation. The rate of oxygen incorporation increases logarithmically with increasing oxidation time. The changes in interatomic distances during the oxidation also provided valuable insights into understanding the evolution of bond formation between atoms. The Co−O interatomic distance is relatively constant from the beginning of the bond formation, whereas the Co−Co interatomic distance increases roughly parabolically as a function of oxidation time. We also found that the pure CoO NP sample based on XRD was not a pure phase discovered by the XAS analysis.
Q. Why is x-ray absorption spectroscopy such a powerful tool for studying chemical transformations in nanostructured systems?
A. XAS is an ideal characterization method for NPs that are undergoing chemical transformation since they usually contain a significant amount of intermediate or disordered structure which cannot be observed from X-ray diffraction.
Understanding chemical transformations in nanoparticles has become increasingly important as novel uses for these materials in emerging energy technologies continues to grow. Thus, it has become equally important to have high quality facilities with the tools needed to study these processes in great detail.
References:
[1] Don-Hyuang Ha, Liane M. Moreau, Shreyas Honrao, Richard G. Hennig, and Richard D. Robinson, “The Oxidation of Cobalt Nanoparticles into Kirkendall-Hollowed CoO and Co3O4:
The Diffusion Mechanisms and Atomic Structural Transformations” The Journal of Physical Chemistry C, 117, 14303 (2013).
Submitted by: Matthew J. Ward, CHESS, Cornell University
12/12/2013
