X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18
As you read this, look around and count the number of items you can see that are made of glass. Glass is everywhere, so it might come as a surprise that it is poorly understood. Most materials freeze at a well-defined temperature into a mass of tiny crystals. Glass is different – over a narrow range of temperature, known as the glass transition, the melt solidifies but retains an atomic structure that is very similar to that of the liquid melt. In fact, the 125th anniversary issue of Science magazine identified the nature of the glass transition as one of the great outstanding mysteries driving basic scientific research [1]. Nobel laureate Philip Anderson has said that “The deepest and most interesting unsolved problem in solid state theory is probably the nature of glass and the glass transition” [2]. Now a group of researchers from Harvard University, led by Professor Joost Vlassak, and the Cornell High Energy Synchrotron Source have combined efforts to develop new experimental capabilities with the potential to provide new insights into the nature of the glass transition [3].
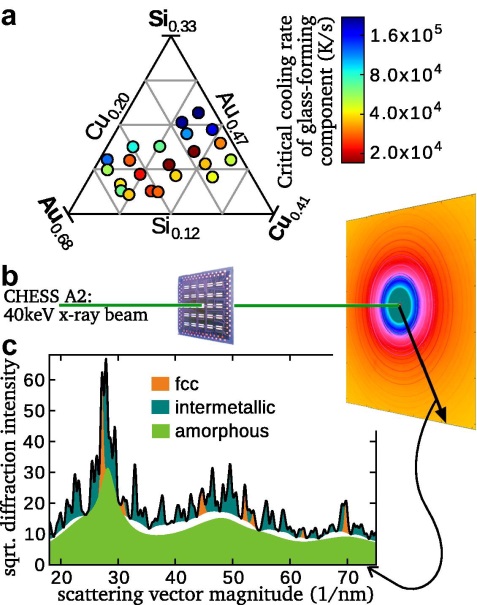
Figure 1.
Vlassak and his team studied gold-based metallic glasses, which are of particular interest due the distinctive properties of Au-rich alloys, such as high corrosion resistance and superior electrical properties. The majority of ternary glassy systems require very high cooling rates to form, and are difficult to study with traditional calorimetry. Vlassak and his colleagues developed nanocalorimeters based on thin film devices, which allows the study of fundamental properties of metallic glasses at ultrafast heating and cooling rates. They then deposited a composition-spread thin film on an array of such devices, which allowed them to study how these properties change with the composition.
X-ray diffraction was needed to determine the amount of material in glassy or crystalline phases, but an intense monochromatic x-ray source was required to study the vanishingly small quantities of material in the nanocalorimeter devices. The group used intense synchrotron radiation from the Cornell High Energy Synchrotron Source to study the fraction of material vitrified into a metallic glass over a range of cooling rates. By combining these experiments, they were able to map the critical cooling rate for vitrification over an array of 22 compositions in the Au–Cu–Si system and characterize the phase changes at unprecedented heating rates for metallic glass systems. They conclude that these new experimental capabilities “offer unique opportunities to explore the asymmetry of crystallization kinetics upon heating and cooling, to directly correlate the effect of cooling rate on the glass transition and crystallization upon heating, and to narrow the vast gap between conditions in simulations and typical experiments.”
References:
[1] "So Much More to Know …," Science, vol. 309, pp. 78-102, 2005.
[2] P. W. Anderson, "Viewpoint: The Future," Science, vol. 267, pp. 1615-1616, 1995.
[3] J. M. Gregoire, P. J. McCluskey, D. Dale, S. Ding, J. Schroers and J. J. Vlassak, "Combining combinatorial nanocalorimetry and X-ray diffraction techniques to study the effects of composition and quench rate on Au–Cu–Si metallic glasses," Scripta Materialia, vol. 66, pp. 178-181, 2012.
Submitted by: Darren Dale, CHESS, Cornell University
4/23/2013
