X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18

Jeney Wierman, biophysics graduate student
Jennifer (Jeney) Wierman, a rising star in biophysics at Cornell University and a member of the research group of Sol M. Gruner, was recently awarded the Margaret C. Etter Student Lecturer Award in the Synchrotron Radiation Scientific Group by the American Crystallographic Association. The award's namesake, Professor Margaret C. Etter (1943-1992), was a major contributor to the field of organic solid-state chemistry, and crystallography. In both academic and industrial realms, Etter found that involvement in the scientific community was very important, valuing relationships with colleagues and students. She focused much of her attention on mentoring and peer support. Following in this spirit, an award was established in her name in 2002 to recognize students for scientific innovation in crystallography.
As a graduate student Jeney has been working since 2011 at the Cornell High Energy Synchrotron Source (CHESS) and the macromolecular diffraction facility at CHESS (MacCHESS). The majority of her research has focused on improving data collection of x-ray scattering for protein crystallography measurements. In 2012 she began working with graphene, which are thin sheets of one atom thick layers of carbon. Before coming to Cornell, Jeney earned degrees in both biology and physics. She initially saw herself going into medicine but found research a more enjoyable atmosphere for her work. CHESS and MacCHESS are facilities that focus on education, open to innovation and supportive of teaching. Jeney reflects that, “at CHESS, people have an incredible sense of ingenuity and readiness to collaborate with others.”
Only since 2009 have techniques for fabricating graphene become available. Graphene has remarkable properties of being ultimately thin, yet strong enough to withstand atmospheric pressure, is optically transparent, and is impermeable to gases (including helium) and unreactive liquids. These properties make graphene useful for applications such as scatterless x-ray beamline windows and holding specimens in pockets of liquid inside high-vacuum environments of electron microscopes, to name a few. Jeney’s work forged new ground by demonstrating that graphene will have important impacts on protein crystallography.
Jeney was invited to present her work on introducing and applying graphene to protein crystallography at the 2013 annual American Crystallographic Association meeting held in Honolulu, Hawaii, July 20 -24. This work was recently published in the Journal of Applied Crystallography [1].
Protein crystallography is a method of determining molecular structure by monitoring scattered x-ray patterns. By analyzing the way high energy x-rays interact and change direction (e.g. are diffracted) by a protein, the molecular structure of the protein can be revealed. Two major challenges of this technique are damage to the protein and low signal-to-noise in the diffraction patterns. Cooling the samples to very cold (cryogenic) temperatures and performing very fast (lasting fractions of a second) measurements help prevent much of the damage caused by intense high energy x-ray beams.
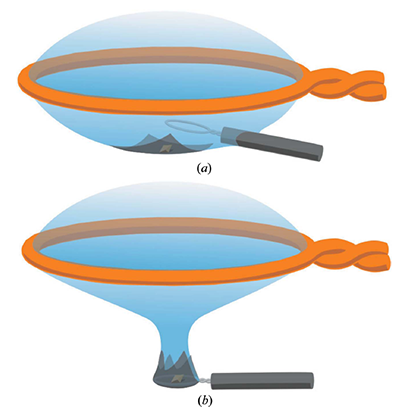
Graphic depicting how the graphene and crystal are mounted on a Hampton cryoloop.
Jeney uses graphene in an attempt address the second of these problems: improving the signal-to-noise ratio in diffraction patterns [1, 2]. The best way to achieve this is to reduce the scattering of x-rays by anything that is not the protein (often call “background scattering”). As a single layer a carbon atoms, graphene is too thin to significantly scatter, yet it is still relatively strong. Jeney's work demonstrates that it is feasible and practical to handle graphene, and that graphene can be used to replace or improve the standard specimen holders to achieve vanishingly small background scattering. Jeney’s work also demonstrated that wrapping a protein crystal in graphene could prevent specimens from dehydration.
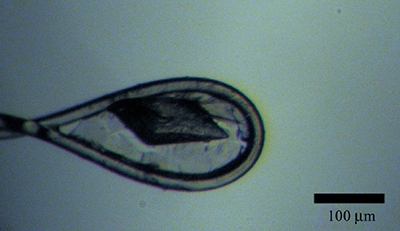
Cryocooled thaumatin crystals wrapped in three layers of graphene on a Hampton cryoloop.
The results of Jeney’s work have raised a lot of interest in the protein crystallography community. The talk generated interest among scientists from several different areas, possibly initiating collaboration on a variety of applications. The potential for reducing background scatter by replacing sample mounts with graphene is a novel breakthrough in protein crystallography measurements. Her work points out that graphene could have applications in many areas of science where there is need to hold and handle specimens without overwhelming the measurements by the presence of a substrate, such as scattering from weakly diffracting liquids and disordered materials to providing flat backgrounds for objects studied by coherent x-ray diffraction imaging.
References:
[1] J. L. Wierman, J. S. Alden, C. U. Kim, P. L. McEuen, and S. M. Gruner. Graphene as a protein crystal mounting material to reduce background scatter. Journal of Applied Crystallography, 46:1–7, 2013.
[2] C. U. Kim, J. L. Wierman, R. Gillilan, E. Lima, and S. M. Gruner. A high-pressure cryocooling method for protein crystals and biological samples with reduced background X-ray scatter. Journal of Applied Crystallography, 46:234–241, 2013.
Submitted by: Margaret Koker, CHESS, Cornell University
10/17/2013
