X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18
Organic electronics is being extensively studied for its potential to create flexible displays [1] and sensors [2]. The current best performance materials involve soluble aromatic molecules with extended π-orbitals. A classic example of such a molecule is TIPS-pentacene with a pentacene backbone and two bulky tri-isopropyl silyl ethynyl (TIPS) side groups that render the molecule soluble in toluene. A feature of this class of materials is low lattice symmetry and polymorphism, i.e., depending on the crystallization conditions, different crystal structures and types of molecular packing may form. The distance between the p-orbitals is all-important for device performance - the closer the better. Since the charge carriers have to tunnel from one molecular π-orbital to the next, even variations in distance of a few tenths of an Angstrom can make a huge difference in charge mobility. Hence controlling the formation of the optimum polymorph has become a grand challenge in the field of organic electronics. An international research team with members from Stanford University, King Abdullah University of Science and Technology (KAUST, Saudi Arabia), and Cornell University recently reported a convenient method to control polymorph formation [3].
Gaurav Giri, a graduate student in Zhenan Bao's group at Stanford University, recently discovered that using a coating technique, termed "solution shearing" that was developed earlier in his lab, a new polymorph of TIPS-pentacene could be obtained with more than five times as large a mobility as the equilibrium polymorph [4]. In solution shearing, a drop of solution is spread at a prescribed speed with a coating blade. However, the exact formation mechanism of the new polymorph remained in the dark, as only ex-situ characterization of ready-made device films could be done at Stanford. In order to glimpse into the actual formation of such films, the Stanford group joined forces with Aram Amassian's group at the King Abdullah University of Science and Technology (KAUST, Saudi Arabia). Amassian and his postdoc, Ruipeng Li, had previously collaborated with staff scientist Detlef Smilgies at the Cornell High-Energy Synchrotron Source (CHESS), in order to develop novel real-time in-situ multiprobe experiments [5]. However, the new experiments demanded a completely novel set-up to be designed for use at CHESS D1 station.
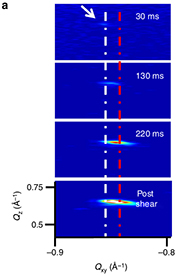
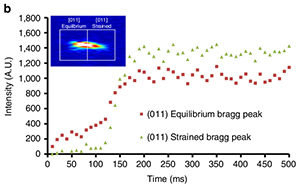
Figure 1. Crystallization of TIPS-pentacene during solution shearing. (a) Time evolution of the (011) Bragg reflections, which shows the formation of the equilibrium (white dash-dotted line) and sheared (red dash-dotted line) polymorphs. (b) Time-lapsed integrated intensities of equilibrium polymorph (red) and strained polymorph (grey). Note that the equilibrium polymorph forms first.
Over various trial runs Giri, Li, and Smilgies developed an experimental setup that did the seemingly impossible: They designed a miniature knife coater that replicated the coating parameters of the much larger Stanford coater, they prepared an x-ray microbeam half the width of a human hair, and finally employed a new high-speed x-ray camera that had just been purchased by CHESS [6]. They found that for coating conditions to obtain the new TIPS-pentacene polymorph which require high coating speed and elevated temperature of the substrate, the fast x-ray detector needed to run at 100 frames per second. Thanks to the low background of this pixel array detector produced by the Swiss company Dectris and the high-flux D1 microbeam, there were just enough x-ray photons around to watch the molecules crystallize (Figure 1).
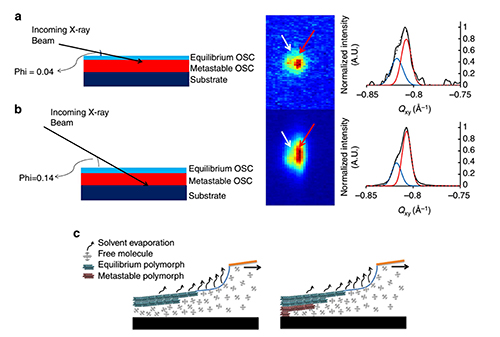
Figure 2. Depth-resolved x-ray scattering. (a) By varying the incident angle of the x-ray beam, its penetration depth can be controlled, thus making it sensitive to the film surface at shallow angles. (b) At higher incident angles the full film is probed. The resulting (011) reflections and their integrated intensities of both polymorphs are shown to the right. These results show that the equilibrium polymorph first forms close to the film surface yielding a crystalline skin layer that entrains the remaining liquid. The entrained liquid solution successively crystallizes in the strained polymorph upon further evaporation of the solvent.
By varying the incident angle of the x-ray beam the team could probe what happens at the film surface and at the interface with the substrate. These measurements provided the important clue how the strained polymorph was formed: first, the equilibrium polymorph is formed at the surface of the solution film. Then the remaining entrained liquid film forms the strained polymorph at the solution-substrate interface, where it is gives rise to the observed high charge mobility within an organic field-effect transistor structure (Figure 2). As entrainment seemed to play a significant role in the polymorph selection, Giri decided to study in-situ solution shearing using a series of solvents with increasing molecular size, and he showed that, consistent with the above interpretation, larger solvent molecules are more effective in obtaining the sheared polymorph.
Paulette Clancy and her student Kristina Lenn at the Cornell School of Chemical and Biomolecular Engineering performed atomically explicit computer simulations that supported the interpretation of the experimental findings. They modeled ten different solvents used in the experiments and showed that the larger the solvent molecule, the more it destabilizes the equilibrium polymorph in favor of other metastable polymorphs. This is particularly evident in its effect on the angle gamma that helps to define the geometry of the unit cell, which they showed is a particularly "stiff" (i.e., defining) parameter in the energy landscape. The effect of the size of the solvent on the molecules to distort the molecular configuration of the surrounding TIPS-pentacene molecules is shown in Figure 3.
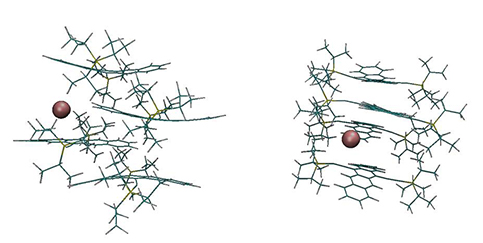
Figure 3. Molecular simulations of the TIPS-pentacene molecule (shown in green) in the presence of even a single solvent molecule (shown as a brown sphere for simplicity) show that the solvent molecule has the ability to significantly bend the acene backbone in the surrounding organic semiconductor molecules, contributing to the destabilization of the equilibrium polymorph.
Preparing TIPS-pentacene thin films by solution shearing has yielded unprecedented control of crystal structure and polymorph selection. The explanation for this effect is rooted in the entrainment of the molecule by the solvent and the complex potential energy landscape of crystalline TIPS-pentacene yielding a variety of metastable structures close to the equilibrium polymorph. The solution shearing method is relatively simple to implement, uses much less material than standard laboratory techniques such as drop casting and spin-coating, and is adaptable to industrial roll-to-roll processing to yield efficient production of high-performance devices [7].
References:
[1] Gerwin H. Gelinck et al.: "Flexible active-matrix displays and shift registers based on solution-processed organic transistors", Nature Materials 3, 106-110 (2004).
[2] Dion Khodagholy et al.: "In vivo recordings of brain activity using organic transistors", Nature Commun. 4, 1575 (2013).
[3] Gaurav Giri et al.: "One-dimensional self-confinement promotes polymorph selection in large-area organic semiconductor thin films", Nature Commun. 5, 3573 (2014).
[4] Gaurav Giri et al.: "Tuning charge transport in solution-sheared organic semiconductors using lattice strain", Nature 480, 504-508 (2011).
[5] Aram Amassian et al.: "Solvent Vapor Annealing of an Insoluble Molecular Semiconductor", J. Mater. Chem. 20, 2623–2629 (2010).
[6] Detlef-M. Smilgies et al.: "Look fast: Crystallization of conjugated molecules during solution shearing probed in-situ and in real time by X-ray scattering", PSS-RRL 7, 177-179 (2014).
[7] Detlef-M. Smilgies: "In-situ real-time x-ray scattering for probing the processing-structure-performance relation", Mat. Res. Soc. Symp. Proc., submitted (2014).
See also the Stanford-KAUST press release:
http://www.eurekalert.org/pub_releases/2014-04/ssoe-gst041514.php#
and the Cornell Chronicle article, 05/14/14:
http://www.news.cornell.edu/stories/2014/05/x-rays-computer-simulations-reveal-crystal-growth
Submitted by: Detlef Smilgies, CHESS, Cornell University
05/05/2014
