X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18
Organic molecules are proving themselves attractive and promising alternatives for electrical energy storage applications. Quinones, in general, and anthraquinones, in particular, are especially attractive due to their ability to reversibly exchange multiple electrons per formula unit. When used as the active electrode material in a real lithium-ion battery (LIB), crystalline anthraquinone (in powder form) reversibly changes crystal packing as a function of state-of-charge (redox state), with a well-defined voltage plateau appearing concomitantly with new structural phases.
Operando powder X-ray diffraction (XRD) is a powerful method for screening the structural stability of organic cathode candidates and for understanding electrochemically-induced structural transformations within organic molecular crystals. High energy x-rays are useful in particular for their ability to penetrate through battery cases and internal structures. In work performed at beamline A2 as a Cornell graduate student, lead author Katharine Silberstein (now a CHESS/Chemistry postdoctoral associate) and coworkers explored the electrochemical lithiation-induced polymorphism of anthraquinone and three related derivatives using coin cells specially designed for X-ray transmission [1]. In this work, four anthraquinones (grouped by differing electron-withdrawing/donating ability) were selected to study by operando powder X-ray diffraction (see figure). The different electronic and structural aspects of these four derivatives impact the coordination of lithium to the reduced species and, therefore, impact the 3D packing arrangement of the lithiated structures.
To the best of current knowledge, the cyclability of three of the four molecules presented in this manuscript has not yet been reported in the literature. They observed clear crystalline changes in tandem with electrochemical action for four anthraquinone derivatives in LIBs. The material that had the most stable cycling performance showed clear evidence of diffraction peaks fading and other peaks growing during charge/discharge cycling. The fact that they see new peaks at low voltage gives credence to the claims that ion-pairing is occurring between lithium cations and anthraquinone (di)anions.
One of the longer term goals of the work is to fully describe these newly formed molecular crystals, so as to be able to propose mechanisms by which the quinone moieties charge-compensate. This analysis will serve as a model for studying organic charge storage within crystalline small-molecule candidates.
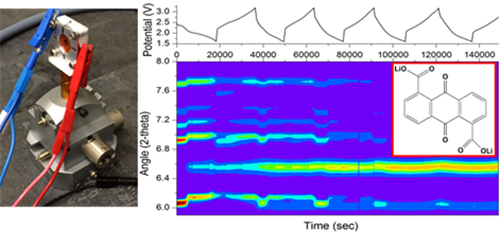
Figure: Using a coin cell with X-ray transparent windows (left), X-ray diffraction patterns of organic molecular crystals changing orientation as a function of electrochemical potential were collected in real time (right).
Reference:
[1] Katharine E. Silberstein, James P. Pastore, Weidong Zhou, Rebecca A. Potash, Kenneth Hernández-Burgos, Emil B. Lobkovsky, and Héctor D. Abruña, “Electrochemical lithiation-induced polymorphism of anthraquinone derivatives observed by operando X-ray diffraction”, Phys. Chem. Chem. Phys., 2015, 17, 27665-27671 DOI: 10.1039/C5CP04201A.
Submitted by:
Katharine Silberstein, CHESS, Cornell University
11/11/2015
