X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18
We often consider bystanders and spectators unimportant, however in chemical reactions they can make a difference, as shown in a recent paper by David Moore and coworkers [1], that just came out in Journal of the American Chemical Society (JACS). Object of the study were inorganic-organic halide perovskites, currently one of the hottest materials in non-silicon solar cells with efficiencies over 20%, rivaling amorphous silicon. Halide perovskites also outperform other types of solar cells such as dye-sensitized or bulk heterojunction organic solar cells that have been studied intensely over the past decade, while the inorganic-organic perovskites are the new kids on the block.
A perovskite features a cubic lattice or slightly distorted cubic lattice, with one cation in the center, a second type of cation on the corners of the cube and anions in the center of the cube faces forming a stoichiometry of ABX3. In the case of halide perovskites there is a lead cation in the center, methylammonium cations on the corners, and a halide as anions (I, Br, or Cl). Now what is special about such a complex material? First of all, it can be relatively easily made from inexpensive precursors, and the process requires temperatures of only 100°C, much lower than temperatures typically used in silicon processing. However, the details of perovskite formation were shrouded in mystery before the new study. First of all, the magic mixture of components has a different stoichiometry from the final material. So what is the role of the ions that are not involved in the end product, the so-called spectator ions?
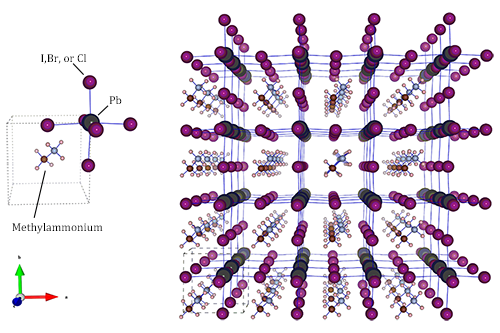
Figure 1: Structure of the inorganic-organic perovskite family of the methylammonium lead halides [2], unit cell (left) for the pseudo-cubic structure and 4 X 4 X 4 supercell (right). Halide ions can be chloride, bromide, and iodide, as shown above.
Moore started comparing different precursor mixtures all yielding the required perovskite; however, the time scales of the reactions varied considerably. This led him to embark on a detailed study of the formation kinetics, making use of in-situ real-time x-ray diffraction data obtained at CHESS D-line. Key to the experiment was a temperature-controlled in-situ hot stage and a fast but sensitive x-ray area detector. The perovskite material was found to be extremely prone to radiation damage during processing; hence only images with brief exposures were taken at larger time intervals.
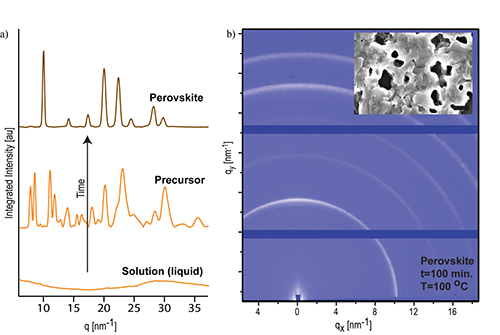
Figure 2: Integrated 1D diffraction plots showing the transformation to the perovskite via a solid-state, crystalline intermediate (Precursor), the time between the plots varies depending on the lead salt used (a). Wide-angle x-ray diffraction image of the fully formed perovskite after 100 min of thermal treatment at 100°C. The insert shows an electron microscope image of the obtained material (b).
The experiments performed at CHESS, in addition to providing quantitative kinetics data, helped answer several other key questions regarding these perovskites. Figure 2a shows that the crystallization is actually a two step process in which the perovskite is preceded by a solid-state, crystalline intermediate; this told the researchers that the perovskite formation was actually a solid state transformation. More importantly, the results show that changing the spectator ions is a kinetic effect, as opposed to a thermodynamic effect, providing insight into how the transformation can be controlled. Leveraging the results of this study should allow for better film and crystal growth by judicious choice of the lead salt reagent; one example of this is the recent report in which lead acetate is used [3] resulting in much smoother films with complete surface coverage as seen in figure 3c.
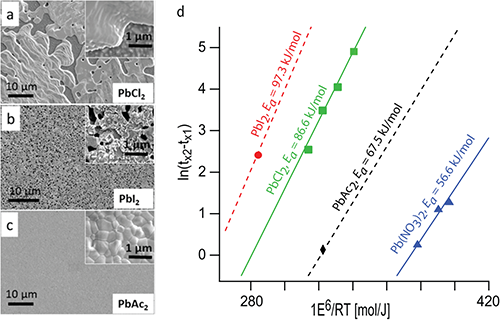
Figure 3: SEM images of methylammonium lead iodide made using PbCl2 (a), PbI2 (b), and PbAc2 (Ac=acetate) as the lead source. Arhennius plot for all four lead sources with the extracted activation energy (d). a, b and c adapted from reference [3].
References:
[1] David T. Moore, Hiroaki Sai, Kwan W. Tan, Detlef-M. Smilgies, Wei Zhang, Henry J. Snaith, Ulrich Wiesner, and Lara A. Estroff, "Crystallization kinetics of organic-inorganic trihalide perovskites and the role of the lead anion in crystal growth", JACS DOI 10.1021/ja512117e (2015).
[2] Giacomo Giorgi, Jun-Ichi Fujisawa, Hiroshi Segawa, and Koichi Yamashita, "Small Photocarrier Effective Masses Featuring Ambipolar Transport in Methylammonium Lead Iodide Perovskite: A Density Functional Analysis", J. Phys. Chem. Lett., 4, 4213–4216 (2013).
[3] Wei Zhang, Michael Saliba, David T. Moore, et al., "Ultrasmooth organic–inorganic perovskite thin-film formation and crystallization for efficient planar heterojunction solar cells", Nature Communications DOI 10.1038/ncomms7142 (2015).
Submitted by:
Detlef Smilgies (CHESS) and David Moore (Cornell Materials Science & Engineering)
Cornell University
02/05/2015
See also related Cornell Chronicle article here:
"Researchers report better solar cells through chemistry"
