X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18
Through diligent chemistry work on purifying small gold nanoparticles and then reintroducing a variety of additives, Yixuan Yu and his fellow students from the Korgel group at the University of Texas at Austin have found the key ingredient to the mysterious phenomenon of cooperative Ostwald ripening. Their results have just appeared in the ACS journal Langmuir and were highlighted as ACS Editors' choice [1]. Ex-situ films were characterized with transmission electron microscope (TEM) at the University of Texas. In-situ grazing-incidence small-angle scattering (GISAXS) data were collected at CHESS D-line. For the latter experiments the sample temperature could be controlled between room temperature and 250°C with a heating block.
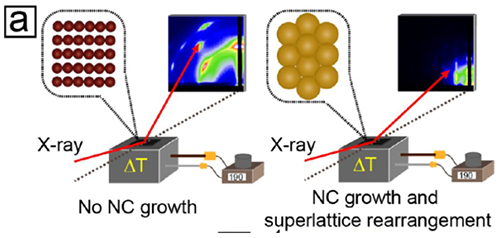
Figure 1: Highly purified gold nanocrystals remain in the BCC phase, until sintering to a 3D porous network occurs above 200°C (left). Addition of additives containing halide ions triggers the formation of binary superlattices of original and fused gold nanocrystals (right).
In regular Ostwald ripening – think of the condensation of water vapor on a cold glass slide – larger drops grow at the expense of smaller droplets which get swallowed up by their larger peers. However, this statistical process results in a random drop pattern at all times. In recent work, also performed by the Korgel group, it was found that small gold nanoparticles of 1.9 nm diameter behave principally different when heated [2,3]: At room temperature the particles already form a BCC lattice. When heated above 130°C, particles fuse in a cooperative way, maintaining ordered lattices as some neighboring particles fuse while others don’t. All in all the system undergoes 3 different phases known from binary superlattices [4], all obtained from the same starting material by simple heating.
However, when particles were carefully purified to remove all synthesis educts, the researchers found that such particles do not form any of the binary superlattice structures seen before – they merely fuse to a random 3D network above 200°C. Reintroducing small quantities of surfactants used in synthesis, the group found that the gold particles fused again, when a small amount of tetraoctylammonium bromide (TOAB) was added. A similar behavior was obtained with other additives containing Cl–, Br–, or I– ions, while additives containing thiol, amine, carboxyl, hydroxyl, and other functional groups did not show any effect. Hence the key seems to be the presence of halide ions introduced by the successful additives.
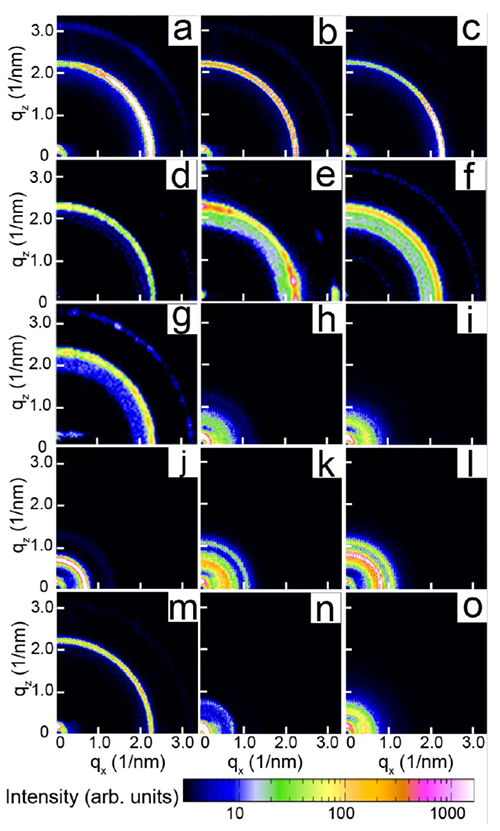
Figure 2: Small-angle scattering from gold nanocrystal superlattices using non-halide-containing (a-g) and halide containing (h-o) additives at 190°C. Only halide-containing additives produce binary superlattices of partially fused gold nanocrystals. For details see [1].
Our image of cooperative Ostwald ripening emerges as follows: Small gold nanoparticles have a strong melting point depression compared to solid gold. The dodecane thiol ligands stabilizing the particles in solution do not come off before reaching 200°C, so that particles can merge or sinter, as we saw for the purified material. However, in the presence of halide ions, ligands are already displaced at lower temperatures just above 130°C. This permits the fusion of particles already at lower temperature which facilitates the organized pattern formation as seen with TEM and GISAXS. While not all mysteries of cooperative Ostwald ripening are resolved at this point, the method provides a facile route to preparing a number of binary superlattices comprising large and small gold nanoparticles from the same starting material.
References:
[1] Yixuan Yu, Brian Goodfellow, Michael Rasch, Christian Bosoy, Detlef M. Smilgies, and Brian Korgel: "The Role of Halides in the Ordered Structure Transitions of Heated Gold Nanocrystal Superlattices", Langmuir (online 6/2015). DOI: 10.1021/acs.langmuir.5b01498
[2] Brian W. Goodfellow, Michael R. Rasch, Colin M. Hessel, Reken N. Patel, Detlef-M. Smilgies, and Brian A. Korgel: "Ordered Structure Rearrangements In Heated Gold Nanocrystal Superlattices", Nano Lett. 13, 5710–5714 (2013).
[3] http://news.chess.cornell.edu/articles/2013/KorgelFused11012013.html
[4] Elena V. Shevchenko, Dmitri V. Talapin, Nicholas A. Kotov, Stephen O’Brien, and Christopher B. Murray: "Structural diversity in binary nanoparticle superlattices", Nature (Letters) 439, 55-59 (2006).
Submitted by:
Detlef Smilgies, CHESS, Cornell University
06/19/2015
