X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18
Mitochondrial cytochrome c peroxidase (CcP) binds to cytochrome c (Cc) to break down hydrogen peroxide to water. This reaction is a series of steps that involves heme-oxygen chemistry and long-range electron transfer (ET) (Figure 1). Firstly, hydrogen peroxide reacts with Fe(III) heme of CcP to form an Fe(IV) iron oxo species [Fe(IV)=O], and oxidizes its nearby tryptophan 191 (W191) to a radical cation (W•⁺). Secondly, the presence of W•⁺ facilitates the transfer of electrons from Cc proteins when Cc Fe(II) is oxidized, causing the reduction of CcP W•⁺ to W191. The CcP Fe(IV)=O then re-oxidizes W191 back to its radical cation state, resulting in the eventual formation of Fe(III) and water.
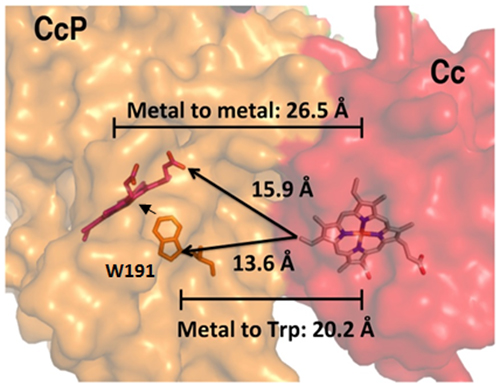
Figure 1: W191 mediates long-range ET between the two heme centers (~16Å), resulting in two short hops instead of one long-range leap.
Since aromatic residues play a crucial role in mediating the long-range ET of many biological redox systems (Payne et al.), the authors investigated the effect of substituting W191 for other residues in the CcP:Cc complex. CcP:Cc was chosen because it is a standard model system for studying long-range ET between proteins (Volkov et al.).
Since W191 is a residue that is critical in the reaction in the CcP:Cc system, three CcP W191X variants were created (where X = Y, F or G; tyrosine, phenylalanine or glycine respectively) to test for the effect of amino acid substitution on ET. Crystal structure complexes of CcP W191X variants bound to Cc showed similar 1:1 association modes and heme pocket conformations (Payne et al.). Unlike the wild-type (WT) W191 that formed a hydrogen bond with aspartate 235 (D235), none of the aromatic substitutions (Y191 and F191) had any polar contacts (Figure 2). The absence of the indole group in the W191G variant produced a water-filled cavity, where redox-active ligands were added. Ligands that bound in the W191G pocket were structurally disordered and did not form hydrogen bonds with Asp235 either. Furthermore, electron paramagnetic resonance (EPR) spectroscopy showed that only the WT and W191Y variants could form a stable W/Y radical formation (Figure 3).
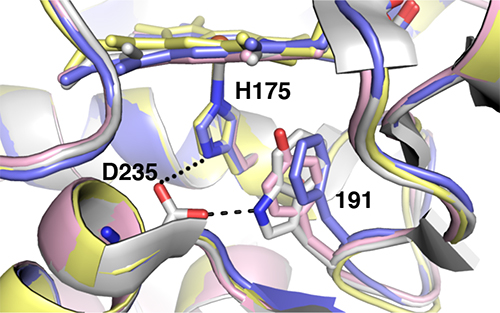
Figure 2: Superposition of the W191X variants. WT in white; W191Y pink; W191F blue; W191G yellow.
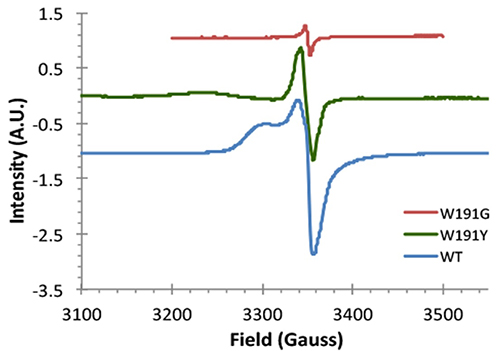
Figure 3: EPR spectra of the CcP variants. Peroxide induced the formation of radicals that was captured by EPR. WT and W191Y signals were significantly larger than the other variants (W191F not shown).
To study how the CcP W191 variants affected the long range ET to Cc, the heme in the CcP variants was substituted with zinc-porphyrin (ZnP), which ET could now be photoinduced. These variants had low ET rate compared to the WT, with the W191G being weaker than the W191F variant. As for the W191Y variant, low net reactivity towards Cc(II) could be due to the inability of: 1) the ZnP•⁺ to produce enough W191Y radical, or 2) the resulting neutral W191Y radical to oxidize Cc(II) effectively.
In conclusion, this study showed that there must be both structure stability and effective redox potential in order to carry out significant long-range ET between the CcP and Cc proteins. At this point it seems that only WT W191 meet these requirements.
References:
[1] Payne TM, Yee EF, Dzikovski B, Crane BR, Constraints on the Radical Cation Center of Cytochrome c Peroxidase for Electron Transfer from Cytochrome c. Biochemistry. 2016 Aug 30;55(34):4807-22.
[2] Volkov AN, Nicholls P, Worrall JA. The complex of cytochrome c and cytochrome c peroxidase: the end of the road? Biochim Biophys Acta. 2011 Nov;1807(11):1482-503.
Submitted by: Teck Khiang Chua, MacCHESS, Cornell University
09/06/2016
