X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18
Kyle Lancaster’s (Cornell C&CB) group has published a study on the contested electronic structure of [Cu(CF3)4]– investigated using UV/visible/near IR spectroscopy, Cu K-edge X-ray absorption spectroscopy, and 1s2p resonant inelastic X-ray scattering.
Trifluoromethyl (CF3) substituents profoundly influence properties of organic molecules and transition metal complexes. Medicinal chemists have recognized the value of the CF3 group for advantageous drug properties including increased physiological longevity, facile blood−brain barrier penetration, and enhanced protein−substrate binding affinities. Consequently, developing reactions that install CF3 on organic frameworks is a major research goal.
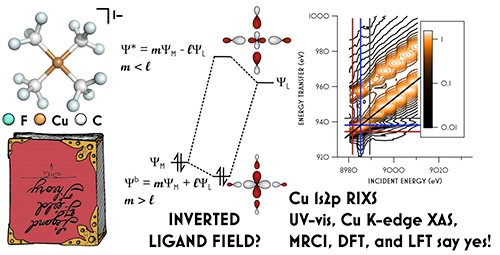
The contested electronic structure of [Cu(CF3)4]1– is suggested in the middle of the above figure. Results obtained by UV/visible/near IR spectroscopy, Cu K-edge X-ray absorption spectroscopy, and 1s2p RIXS measured at CHESS are supported by density functional theory, multiplet theory, and multireference calculations. Together they support a ground state electronic configuration in which the lowest unoccupied orbital is of predominantly trifluoromethyl character. The molecule is illustrated at left, and a RIXS spectrum is displayed at right.
These data, supported by density functional theory, multiplet theory, and multireference calculations, support a surprising ground state electronic configuration with lowest unoccupied orbital predominantly of trifluoromethyl character. The consensus 3d10 configuration features an inverted ligand field in which all five metal-localized molecular orbitals are located at lower energy relative to the trifluoromethyl-centered σ orbitals.
Understanding the electronic structures of transition metal catalysts and reagents is key to controlling their reactivity. The present study sets the stage for development of new trifluoromethylating agents based on earth-abundant Cu that exploit electrophilic rather than nucleophilic reactivity.
Reference:
Walroth RC, Lukens JT, MacMillan SN, Finkelstein KD, and Lancaster KM, "Spectroscopic Evidence for a 3d10 Ground State Electronic Configuration and Ligand Field Inversion in [Cu(CF3)4]1−"
Submitted by: Kenneth Finkelstein, CHESS, Cornell University
02/08/2016
