X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18
Crystal structure of mammalian cysteine dioxygenase. A novel mononuclear iron center for cysteine thiol oxidation.
C. R. Simmons, Q. Liu, Q. Huang, Q. Hao, T. P. Begley, P. A. Karplus, and M. H. Stipanuk
J. Biol. Chem. (2006), Vol. 281, 18723-18733
Cysteine dioxygenase (CDO) is an enzyme that catalyzes the oxidation of the sulfur-containing amino acid cysteine by O2 to form cysteine sulfinate. This is an important step in several critical metabolic pathways; blockage of the oxidation leads to depletion of sulfate and taurine, and accumulation of cysteine, with resulting neurological impairment and other adverse effects.
A number of dioxygenases have been studied; most contain iron in the active site, but in a variety of different environments. These enzymes catalyze cleavage of a C-C bond or hydroxylation of a carbon atom, whereas in CDO it is a sulfhydryl group, -SH, that is oxidized. From its amino acid sequence, CDO was expected to have an overall "jelly roll" β-barrel structure, and it was known to contain Fe, but the geometry of the active site could not be predicted. A recent structure of mouse CDO confirmed the β-barrel fold and revealed the structure of the active site, including an unusual covalent cross-link between a cysteine and a tyrosine residue which make up part of the substrate-binding pocket. However, this structure contained nickel rather than iron, and was catalytically inactive. What is different when Fe is present?
Chad Simmons and co-workers (several of whom are MacCHESS people), can now answer this question. They have used 1.5 Å data collected at CHESS (F2 station) and NSLS to determine two structures of native, Fe-containing, rat CDO, one of just the protein and one with added Cys. SAD phasing of a SeMet derivative and difference Fourier methods, respectively, were used to determine the structures. The geometry of the iron center indeed differs from the nickel center in a small but important way: the Ni is coordinated by three histidine residues and three water molecules, while Fe is tetrahedrally surrounded by three His and just one H2O (a highly unusual environment for Fe). From the structure, the authors propose a two-step mechanism: 1) O2 enters the site, displacing a water molecule and binding to the Fe and a nearby Tyr as a superoxide radical; 2) cysteine arrives and is attacked by the radical to form the cysteine sulfinate product, which then detaches from CDO and floats away.
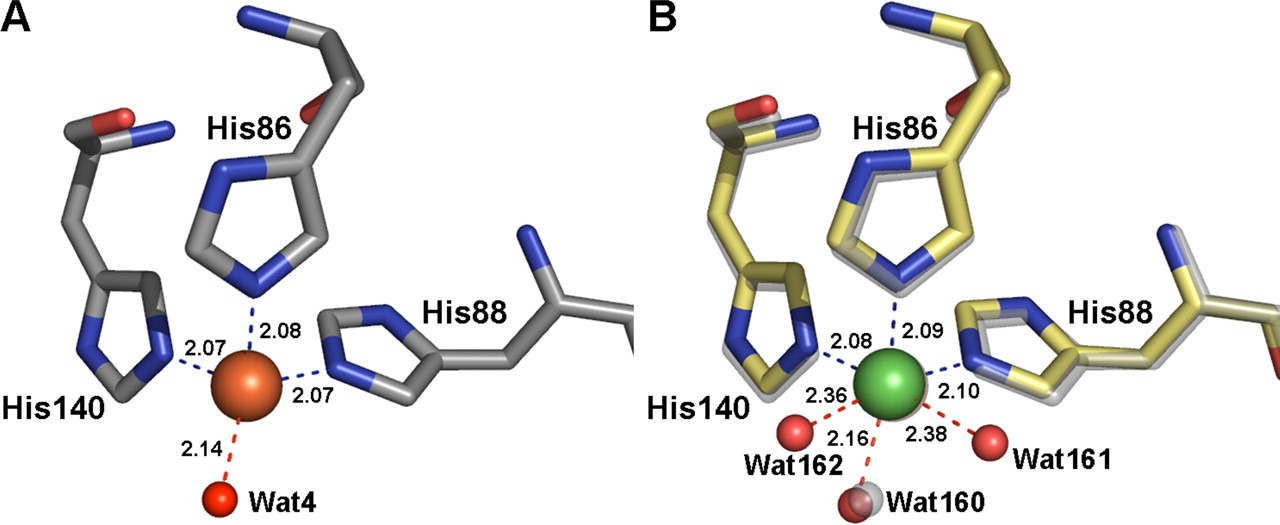
Comparison
of Fe site in rat CDO (left) and Ni site in mouse CDO
(right). Only the species directly coordinated to the
metal atoms are shown.
Fe is brown and Ni is green.
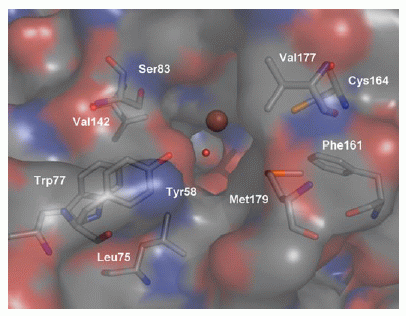
View into the active site of CDO, as an
approaching cysteine molecule would "see" it. The Fe
(brown sphere) is about 8 Å from the surface of the
molecule.
The structure of the CDO-Cys co-crystal was determined in an effort to see how cysteine might bind to the active site. In the electron density map, no Cys was visible in the active site. However, there were clearly structural differences from the native protein. These were not easy to interpret, because they indicated that a mix of native and modified structures was present. Apparently, the added Cys forms a disulfide bond to CDO residue 164, located about 8 Å from the Fe. The bond formation causes a chain of rearrangements in residues lining the substrate-binding pocket, particularly Arg 60 and Met 179, inhibiting access to the active site. This observation is illuminating, in view of the fact that CDO is inhibited by the presence of excess cysteine.
The CDO structure is the first from a new family of metalloenzymes. The mechanism proposed in this paper suggests many further experiments in clarifying how these proteins do their work.
