X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18
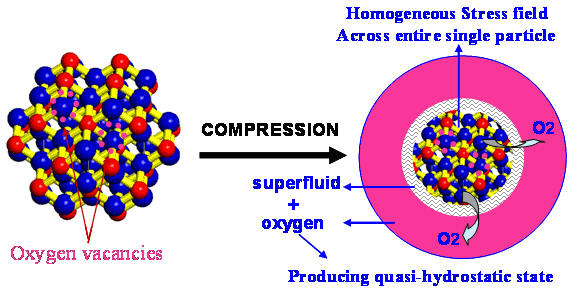
High-pressure causes 3 nanometer
ceria particles to release oxygen. The oxygen
forms a gaseous medium surrounding the nanoparticles and
the vacancy-filled surface layer behaves like a
superfluid.
Contact: E. Fontes (ef11@cornell.edu)
Attention home builders: build your next house using 3 nanometer particles of cerium oxide (CeO2) and it will remain stable up to atmospheric pressures of 65 billion Pascals!
As we learn more and more about the small, nanoscale world around us, scientists and engineers are proposing and building new devices and components from nanoscale materials daily. Before we start building nano-houses and nano-robots, though, lots of challenges and hurdles stand in our way. Foremost among them is a common observation that many materials behave in unpredictable and bizarre ways when they get very small. One such material is cerium oxide, or ceria (CeO2), now widely investigated for future energy applications as either a catalyst or as a storage medium in solid oxide fuel cells. Ceria is also promising for new biomedical applications such as defending cells from reactive oxygen species or protecting normal cells during radiation treatments that destroy tumor cells. Some expect that ceria may have a future in popular topical lotions and sunscreens.
A recent article in the Journal of Physical Chemistry C Letters explains some of the unusual materials properties of nanoceria. The paper, “Anomalous Quasihydrostaticity and Enhanced Structural Stability in 3 nm Nanoceria,” was authored by collaborators Zhongwu Wang, staff scientist at the Cornell High Energy Synchrotron Source (CHESS), Sudipta Seal and Swanand Patil (University of Central Florida), Chang-Sheng Zha (Carnegie Institute of Washington) and Qing Xue (Intel Corp., Chandler Arizona). They present a combined study using high-pressure ruby fluorescence spectroscopy and synchrotron X-ray diffraction to measure the stress and structural stability of 3 nanometer particles of ceria. The paper discusses dramatic differences between 3 nanometer ceria as compared to the bulk material, and document for the first time surprising differences between 3 and 10 nanometer particles.
Bulk ceria is a hard, highly incompressible ceramic, with the common cubic fluorite crystal structure. When loaded into a diamond-anvil cell and squeezed to high pressure, it is not surprising that bulk ceria shows very noticeable deviatoric stress and large pressure gradients – exactly what would be expected when squeezing two hard materials together. Broadening and overlap of lines in the ruby fluorescence spectrum – a sign of significant pressure gradients within the cell – are typical for the ceria samples that have been studied to date.
Very different phenomena are observed when 3 nanometer crystals of ceria are loaded into the diamond cell with no solvent or other pressure medium. First, the ruby fluorescence lines remain sharp at all pressures, indicating an isotropic pressure environment with little or no gradient. This result is exactly what is seen when nanocrystals are surrounded by a hydrostatic pressure medium, such as a liquid. This unexpected “quasi-hydrostatic state” of ceria is maintained up to pressures as high as 28.6 GigaPascals. In addition, the cubic crystal structure remains stable to pressures as high as 65.1 GPa. This stands in stark contrast to the phase transition to an orthorhombic lattice, typically seen around 32 GPa in bulk ceria specimens and at 23 GPa in ceria nanocrystals down to 10 nanometer in size.
What, then, is maintaining the hydrostatic state around the 3 nanometer ceria particles and stabilizing the structure?
The authors explain that the huge differences seen in the smallest 3 nanometer ceria must be related to the specific internal structure of the ultrafine particles. They combine their results with data from others who studied the effects of particle size on lattice parameter and oxygen vacancies. Computer simulations predict that oxygen vacancies occur at the surface of nanoparticles, and that the loss of oxygen weakens the crystalline lattice and results in expansion of the unit cell. They propose that increasing pressure causes oxygen to be released from nanoparticles into the space between them. The released oxygen forms a gas pressure medium that bathes the nanoparticles in a hydrostatic pressure environment. Drawing on prior results showing an enhancement in electrical conductivity in nanoceria, they also suggest that surface vacancies will induce superfluid behavior at the nanoparticles’ surface, similar to the superfluid-like behavior seen in solid helium.
The pressure-driven release of oxygen and the vacancy-induced surface superfluid produce a quasi-hydrostatic state that persists up to a pressure of 28.6 GPa. The anomalous structural stability of the low-pressure cubic phase, up to 65.1 GPa, is closely related to the stress distribution within each surface-modified particle. Using fundamental arguments the authors calculate a critical size for ceria nanoparticles of between 7-18 nanometers – above this size the particles undergo a bulk-like phase transition to the orthorhombic form at a pressure near 20 GPa; below this critical size the low pressure phase remains stable. Seeing 3 nanometer ceria remain stable for an additional 30-40 GPa is remarkable and a surprising reversal of expected behavior – the so-called Hall-Petch relation. This paper presents the first experimental evidence of this effect in nanomaterials.
The authors conclude that these observations on ceria, and arguments explaining the anomalous behavior, add to the growing body of knowledge on nanomaterials and have important applications for scientists and engineers hoping to synthesize novel materials with tunable physical properties.
See Journal of Physical Chemistry C Letters
(archived pdf here)
