X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18
By Marian Szebenyi - July 27, 2010
The protein myosin is essential for the contraction of muscles, and is also used by non-muscle cells to move and reshape themselves. It is even found in single cells such as the amoeba Dictyostelium discoideum (which is known as a slime mold when groups of amoebae gather together to form a "fruiting body" for reproduction). To generate contractile force, myosin molecules must be assembled into filaments, and the assembly is regulated by proteins called myosin heavy chain kinases (MHCK's). These come in several classes, of which the α-kinases are particularly widespread. In Dictyostelium, the α-kinase MHCK A drives disassembly of myosin filaments by phosphorylating three threonine amino acid residues in the "tail" of the myosin molecule, which is composed of α-helices twisted together. The structure of this MHCK A had not been determined directly, but was expected to be similar to that of the related mouse protein TRPM7, for which the conformation of the catalytic domain (TRPM7-CAT) was available. The Jia group (Queen's University, Kingston, Ontario) has now determined the structure of several complexes of the MHCK A catalytic domain (A-CAT), and the results provide new insight into the catalytic and regulatory functions of α-kinases. The work was reported in Science Signaling, where it made the cover:

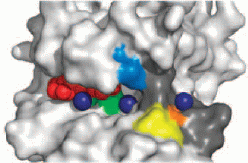
Electrostatic potential (magenta, positive; orange, negative) on the
surface of A-CAT. Bound AMP molecules and phosphate ions (green) are
partially visible in the binding pocket. Mg2+ ions are shown as
purple spheres. "Crystal Structure of the α-kinase Domain of Dictyostelium
Myosin Heavy Chain Kinase A";
Q. Ye, S.W. Crawley, Y. Yang, G.P. Côté, and Z. Jia,
Science Signaling (2010), Vol. 3, ra17
The first complex crystallized, A-CAT plus AMP (ATP is the source of the phosphate group used for phosphorylation; ADP and AMP are what's left after removing one or two phosphates from ATP), had its structure determined to 2.3 Å using data from a laboratory X-ray source and sulfur SAD (single-wavelength anomalous diffraction) phasing. Additional data on this crystal, and data on other crystals, were collected at CHESS F1, and structures of three other A-CAT complexes were determined using molecular replacement. The final resolution ranged from 1.6 – 2.2 Å. Comparison of A-CAT with TRPM7-CAT showed that the core of the catalytic domain is conserved; differences in function among α-kinases are provided by differences in surface features.
Crystallization of A-CAT in the presence of ATP resulted in crystals containing AMP plus a phosphate molecule (and some Mg2+). If a substrate peptide (i. e. a small piece of the myosin chain with which A-CAT reacts) was also present in the crystallization medium, the crystals contained ADP, and the invariant residue Asp 766 of A-CAT was phosphorylated, making it pAsp. This pAsp was exposed to solvent within an active site pocket that could function as a docking site for substrate, and access to it was restricted by a loop whose conformation was altered by binding of Mg2+; hence A-CAT activity could be regulated by changes in Mg2+ concentration. To visualize the conformation of unreacted ATP, structures were also determined for A-CAT in complex with AMPPCP, which is a non-hydrolyzable analog of ATP; and for a mutated, inactive, A-CAT plus ATP. The overall structure of A-CAT was found to be nearly invariant among the different complexes, indicating that no major conformational change is required for MHCK A function.
