X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18
Nanoparticles are nm-sized particles of metals, semiconductors, and insulators surrounded by a shell of organic molecules bound to the surface of the inorganic cores. These organic molecules allow the nanoparticles to be dispersed in liquids, which in turn, facilitates crystallization of the particles into superlattices. Such nanoparticle superlattices constitute a class of synthetic materials with novel optical, chemical, electronic, and magnetic properties.
When the nanoparticles are spherical, they pack into densely packed very symmetric lattices governed by the close packing of hard spheres, i.e., face-centered cubic and the hexagonal close-packed lattices – essentially the same lattices adopted by atoms in metals. However, nanoparticles can be synthesized that are not spherical, e.g., nm-sized cubes, octahedra, and more. The question arises how such non-spherical objects pack. Note – there is no longer a simple analogy with atomic lattices!
James Fang and his coworkers at the Chemistry Department of SUNY Binghamton have recently developed synthesis procedures to obtain a variety of uniformly-sized and -shaped, non-spherical metal-alloy nanoparticles [1]. They were interested in the packing of nanoctahedra, as there is no packing for such objects which can fill space without gaps between the particles. Moreover, the synthesized platinum-nickel particles have interesting chemical properties as catalysts for fuel cells, which is important for alternative energy production. Regular lattices of nanomaterials offer the potential to be used for high-surface-area catalysts exposing the well-defined Pt3Ni (111) facets, which are known to have the largest catalytic activity observed so far.
Studies of such assemblies of nanoctahedra were performed at SUNY Binghamton, Texas A&M University, and CHESS. High-resolution electron microscopy and tomography revealed that the nanoctahedra feature a very unusual tip-to-tip arrangement (Fig 1) - usually a contact between large facets is favored. In this arrangement there is a lot of space available between the metal cores. Ideal octahedra in such a structure would only fill 33% of the available space, and observed was a space filling of about 40%. The remaining space is filled with organic molecules; however, this only accounts for about 67% of the available remaining space. The other 33% may contain solvent molecules or may be simply void volume. This whole arrangement has a surprisingly high symmetry, and forms perfect body-centered cubic lattices, as confirmed with GISXAS measurements at CHESS. Results were recently published in Nanoletters [2].
If solvent ligands can be removed while retaining the observed highly open structure, such materials could serve as extremely efficient catalysts. Experiments are underway to prepare such nanoporous materials.
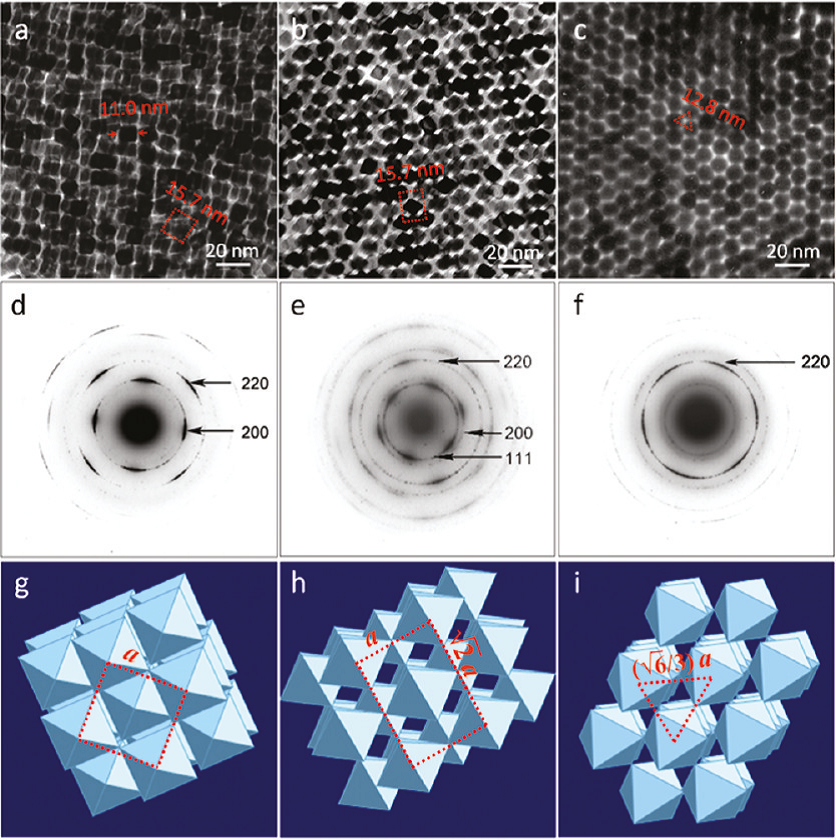
Fig 1: Transmission electron microscopy images (top row) and associated selected area diffraction patterns (middle row). In the bottom row a schematic interpretation of the octahedral packing.
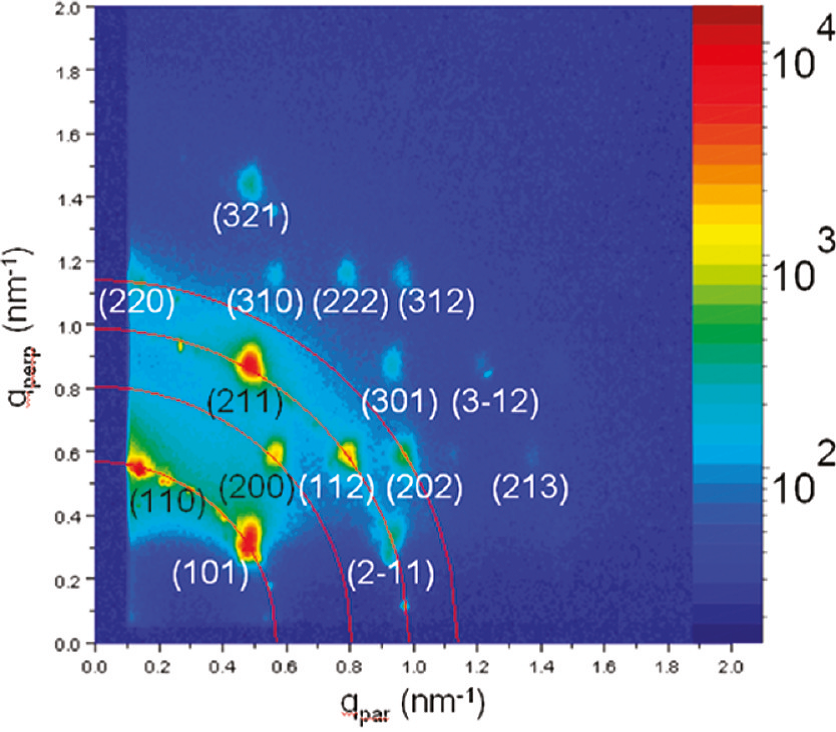
Fig 2: GISAXS image of self-assembled PtNi3 nanoctahedra, as obtained at CHESS D1 station. Observed diffraction spots can be indexed to a body-centered cubic lattice with a lattice constant of 15.7 nm.
References:
[1] Zewei
Wang and James Fang; "Superlattices with Non-Spherical Building
Blocks", Nano Today 5, 390-411 (2010)
[2] Jun Zhang, Zhiping Luo, Zewei Quan, Yuxuan Wang, Detlef-M.
Smilgies, and Jiye Fang; "Low Packing Self-Assembled
Superstructure of Octahedral PtsNi Nanocrystals", Nano Lett. 11, 2912-2918 (2011) (Letter)
Submitted by: Detlef Smilgies, CHESS, Cornell University
