X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18
Rickettsia bacteria are transmitted by the bites of infected ticks and other arthropods, and cause diseases such as typhus and spotted fever. Like many other bacteria, Rickettsia uses the actin cytoskeleton of the host cells to move within a cell and spread from one cell to another. It does this through the agency of a “comet tail” assembled from actin filaments, and inhibition of comet tail formation reduces the virulence of Rickettsia. The bacterial transporter protein Sca2 is required for assembly of comet tails; it functions similarly to the eukaryotic formin proteins in promoting actin filament formation. The structure of Sca2 is important both as a simpler model for the formin-based system and as a possible point of attack for drugs to treat Rickettsia infection.
The Dominguez group (U. Penn.) determined the structure of a fragment, “SCA400” (residues 34-400 of the N-terminal “NRD” domain), of Sca2 from R. conorii. Although comprising less than 1/3 of the full protein, SCA400 was (weakly) active in nucleating actin filaments. Diffraction data were collected at CHESS A1 station, on native and Se-Met derivatized crystals, and the structure was solved using the SAD (Single-wavelength Anomalous Diffraction) method. The final refinement of the native data, to a limiting resolution of 2.18 Å, gave an R/Rfree of 17.9/22.7%.
SCA400 has a crescent shape composed mainly of a repeated helix-loop-helix motif; there are seven copies of the motif, of which three are incomplete. The N-terminal portion of the first helix of each motif is variable in conformation, but the remainder is very consistent, in spite of a lack of sequence conservation across repeats. This fold has not been previously observed, and was particularly unexpected among bacterial transporters, which are generally based on curved β-sheets rather than α-helices.
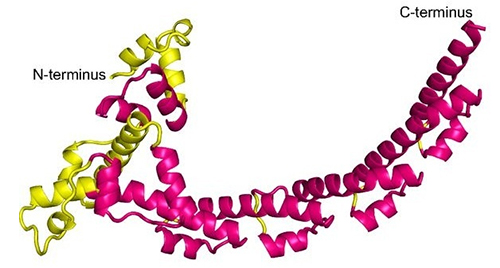
Figure 1. Structure of SCA400. The unique N-terminal region is yellow and the repeating helix-loop-helix units are magenta.
A second domain, “CRD”, of Sca2 is predicted to have a similar conformation to NRD, and the authors suggest a model whereby crescent-shaped NRD and CRD domains fit together to create a ring which promotes assembly and elongation of an actin filament passing through it. A somewhat similar ring is formed by a dimer of the FH2 domains of eukaryotic formins, but the fold of FH2 is entirely different from that of NRD and CRD. The other domains of Sca2, as indicated by mutation experiments, may function similarly to other domains of formin in recruiting actin monomers and interacting with profilin-actin complexes. Bacterial Sca2 and eukaryotic formin represent a striking instance of equivalent functionality achieved through distinct molecular mechanisms.
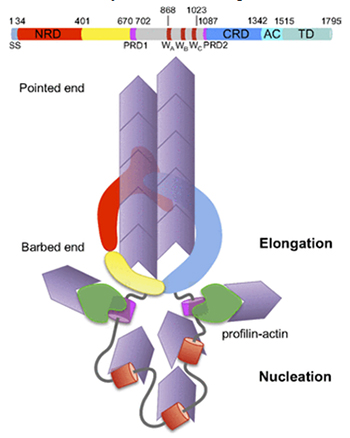
Figure 2. Top, domains in Sca2. Bottom, model for Sca2 surrounding a growing actin filament.
References:
[1] Y. Madasu, C. Suarez, D.J. Kast, D.R. Kovar, R. Dominguez, "Rickettsia Sca2 has evolved formin-like activity through a different molecular mechanism," Proc. Nat'l Acad. Sci. USA 110, E2677-E2686 (2013).
Submitted by: Marian Szebenyi, MacCHESS, Cornell University
08/13/2014
