X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18
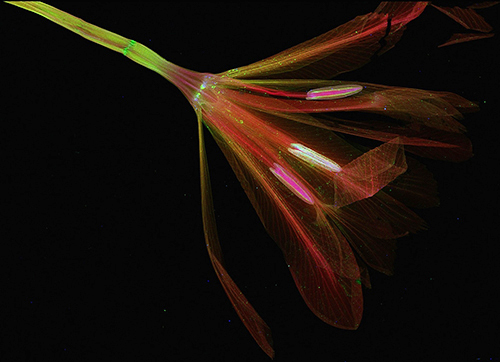
Figure 1: False color representation of elemental distributions of potassium (red), calcium (green) and zinc (blue) in a pressed, dried iris flower, obtained with scanning x-ray fluorescence microscopy. The image has 1920x1320, 0.05 × 0.05 mm2 pixels, with a dwell time of 5 milliseconds/pixel and total scan time of 3.5 hours. (Sample prepared by Lucy E. Woll.)
Over the course of two weeks this past March, scientists from three laboratories commissioned CHESS's most recent purchase, a 384-sensor, energy-dispersive detector known as "Maia". The large number of individual sensors enables much higher count-rates than prior generations of detectors with the same functionality. One use of this combination of energy resolution and high count rate is to map the elemental composition of objects with breathtaking resolution in a much shorter time than was previously possible. Figure 1 represents what the combination of a synchrotron source and this new detector can do. The image is a false-color representation of potassium, calcium, and zinc concentrations in a dried, pressed iris flower. It is striking for both its beauty and the precise information it contains.
The Maia detector is an extremely new, award-winning technology developed by a collaboration between Australia's national science agency, CSIRO, and Brookhaven National Laboratory (BNL) in Upton, NY (https://www.synchrotron.org.au/index.php/news/publications/lightspeed-newsletter/lightspeed-articles/833-csiro-research-award-for-maia-detector-team). Two of the leaders of this collaboration, Robin Kirkham of CSIRO and Pete Siddons of BNL, along with BNL engineer Anthony Kuczewski, traveled to CHESS to join the commissioning run in March, which took place at the G3 station at CHESS. CHESS Staff scientist Arthur Woll led the project, with critical local assistance from a small army of staff and students.
An important application of the Maia detector is scanning x-ray fluorescence microscopy, a well-established technique for obtaining the elemental composition of virtually any material. It works by scanning a sample across a small incident x-ray beam, and detecting secondary x-rays that are re-emitted by atoms at each position that the x-ray beam strikes. The resolution of the scan is determined by the size of the incident beam. For the short term, CHESS will target spatial resolutions ranging from 0.01 to 0.2 mm. For this run, the beam size was 0.05 × 0.05 mm2, with an incident energy of 10.1 keV. Future source and optics upgrades will allow CHESS to employ this technique well below 0.001 mm. The wavelength and intensity distribution of the photons re-emitted from the sample identify which elements are present at each position, but only if there are enough photons – typically thousands – to create a statistically meaningful measurement. The Maia detector allows these thousands of photons to be collected in a short time – about 1 millisecond – enabling faster scan speed.
The image in Figure 1 was collected using this technique. After several days of set up, testing, training, and characterization, the Australian and BNL teams packed up early on March 6th, leaving the CHESS team some time to put the detector through its paces. Among the first samples to be scanned was a pressed iris flower provided by Lucy Woll (Arthur's six-year old daughter). The scan started on March 5th at 11:45 PM, and after a brief analysis the next morning, images like Figure 1 began to appear.
With this image in hand, Arthur and several CHESS staff members sought out other interesting objects to scan. The second week of commissioning, from March 12th-17th, was devoted to performing scans on the wide variety of samples that resulted from this very brief search. Below are some of the images resulting from these scans.
Zak Brown, a CHESS operator and detector expert, contacted friend and entomologist Tarryn Goble, who brought several Asian longhorned beetles. This beetle species is invasive to North America and highly destructive. Tarryn, along with other members of Ann Hajek's laboratory at Cornell, is working to develop a fungus, Metarhizium brunneum F52, as a biological control agent against this species. The results of scans of two pairs of beetle wings as well as the legs and antennae of beetles at differing stages of infection are shown in Figures 2 and 3. The presence of extra, concentrated forms of calcium in the left-most antennae and legs most likely result from the fungus. But both the presence of zinc in the uninfected beetle legs and the distribution of potassium in the wings were a surprise, and stimulate new questions about insect physiology.
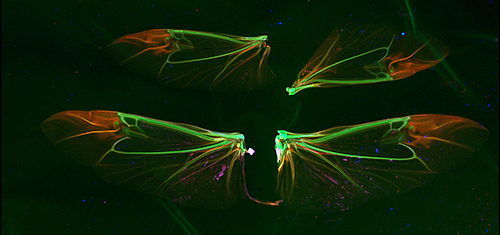
Figure 2: False color representation of the concentrations of elemental distributions within wings from two Asian longhorned beetles. Potassium and zinc concentrations are represented by red and blue, while green represents Compton scattering, which is particularly sensitive to low Z elements, such as carbon. (Sample prepared by Tarryn Goble, Ph.D., post-doctoral associate in the laboratory of Prof. Ann Hajek, in the Cornell Department of Entomology.)
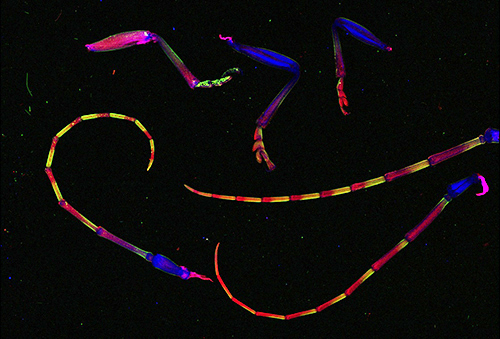
Figure 3: False color representation of concentrations of potassium (red), calcium (green) and zinc (blue) within legs and antennae of three Asian longhorned beetles. (Sample prepared by Tarryn Goble, Ph.D., post-doctoral associate in the laboratory of Prof. Ann Hajek, in the Cornell Department of Entomology.)
CHESS outreach coordinator Lora Hine contacted her colleague Anne Rosenberg at the Lab of Ornithology, who provided a loose breast feather of a Great Blue Heron from the Cornell University Museum of Vertebrates' teaching collection. Such scans show distribution patterns of elements in birds' feathers, (e.g. Figure 4), that may reveal aspects of the birds' chemical environments, such as exposure to pesticides.

Figure 4: False color representation of concentrations of sulfur (red), calcium (green) and zinc (blue) within a breast feather of a Great Blue Heron. (Sample prepared by Anne Rosenberg, Cornell Lab of Ornithology.)
The explicit scientific objective of this effort was to test the instrument, demonstrate its capability, and communicate that capability to potential user-communities. In essence, the goal was to produce eye-catching images to share right away, rather than new science. The surprise was how novel these images appeared to experts in the relevant fields of study.
Arthur sent the iris image from Figure 1 to Karl Niklas, the Liberty Hyde Bailey Professor of Botany in the Cornell Department of Plant Biology, who responded enthusiastically and provided a variety of samples to measure. Two unassuming fern leaflets from the Department's large collection yielded some of the most interesting data of the run, represented in Figure 5. Niklas comments that elemental compositions of plants are traditionally visualized at the scale of individual tissues or even cells. Being able to visualize this composition quantitatively and at "the whole organ level and even the level of an entire plant" is extremely useful, and may provide new understanding of "where and to what extent elements occur in leaves, stems, roots, and reproductive organs."
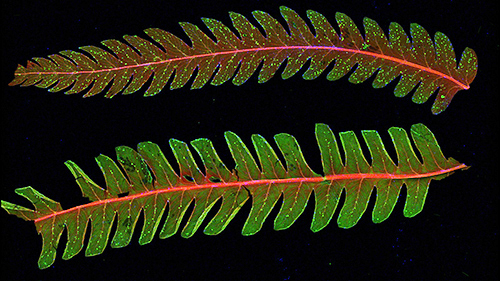
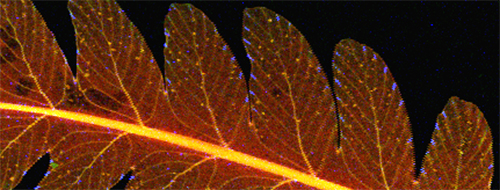
Figure 5: The upper image is a false color representation of concentrations of potassium (red), calcium (green) and manganese (blue) within younger (top) and older (bottom) leaflets taken from a single fern leaf of the cinnamon fern (Osmunda cinnamomea). Both samples exhibit concentrated regions of calcium that are well correlated with leaflet veins. They also exhibit manganese-rich regions located preferentially near the leaflets' outer borders, especially in the right-hand region of the younger leaflet. An enlarged view of this region is shown in the lower image, in which Compton scattering is exchanged for calcium for the green channel. A high degree of correlation between the Compton scattering and potassium distribution leads to an overall yellow hue. But Compton scattering also emphasizes the leaflet structure, from which it can be seen that the manganese regions coincide with the leaflet veins. (Sample supplied by Karl Niklas, professor of botany, Cornell Department of Plant Biology.)
Based upon the capabilities of the Maia and interest of scientists across many disciplines, CHESS anticipates a substantial demand for this instrument. The next commissioning phase will occur in June, and general user access is planned for the Fall of 2014. To facilitate the process, CHESS plans to organize a tutorial workshop to introduce users to both the equipment and to the analysis software. Although users can certainly take their data home with them, the shear data volume – a single scan can easily exceed 100 GB – may present practical difficulties for many users. As a result, CHESS expects many users to perform data analyses remotely using the CLASSE compute cluster. This cluster is a multi-node compute farm maintained by the IT group with a very large, expandable storage array. Off-site access to the software has already been tested successfully.
Further details about scheduling and availability of the Maia, and the tutorial workshop planned for this Fall, will be announced early this summer via the CHESS electronic newsletter and web site.
Submitted by: Arthur Woll, CHESS, Cornell University
04/04/2014
