X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18
The recent realization of X-ray Free Electron Lasers (XFELs) has excited the imagination over what new measurements can be done with the unprecedented peak brightness and short time duration of the pulses. Serial microcrystallography has been developed to pass submicron protein crystals through the x-ray beam during which only a single diffraction image is captured before the specimen is destroyed. In almost all cases the angular orientation of the crystal is not known, so the series of diffraction images cannot be analyzed using existing software approaches.
In addition to protein crystals, there are many other materials systems where the small size, low density, weak contrast, disorder and/or damage all contribute to x-ray image data with either little signal-to-noise or little signal, or both. Or, alternatively, with limited access to x-ray time at XFELs, a natural question to ask is can we extend serial crystallography (or other methods) to be feasible at albeit weaker but readily available synchrotron sources? Such questions are also warranted in light of the development of x-ray detectors with virtually zero background noise that make it possible to record faithfully images with only tens of photons.
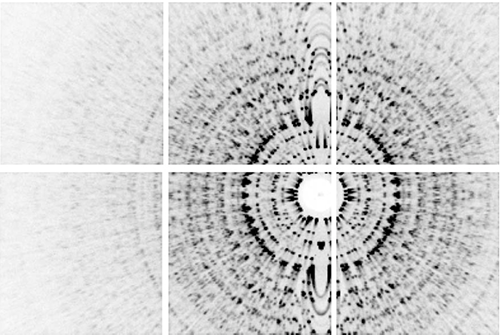
Angle-averaged pattern produced by summing over all frames in the low-fluence data set. The direct beam goes through the center of the beamstop and the rotation axis is vertical. The white gaps in the image are the spaces between the six detector tiles in the 2x3 tiled array of the MMPAD detector.
As a complement to the notion that progress often depends on instrumentation, over the past few years a series of papers from the groups of Cornell faculty Veit Elser and Sol M. Gruner have shown dramatic improvements in data analysis software and algorithms [1,2]. Their latest publication on the “Determination of crystallographic intensities from sparse data” shows how diffraction images with as few as 48 photons per frame, and with no information about specimen orientation, can be used effectively as crystallographic data [3]. In earlier papers they demonstrated that the EMC algorithm can be used to reconstruct structures from sparse radiographic images from noncrystalline structures with unknown orientations [4]. As a proof of principle with the crystallography data, this latest paper reconstructs the three-dimensional diffraction intensity of a 1.35 KDa molecule crystal.
Their efforts suggest that serial crystallography is not limited by the brightness of the x-ray source, and that collection of complete data sets from vanishingly small crystals, or non-crystalline structures, should be possible at existing storage-ring sources.
References:
[1] N. T. D. Loh and V. Elser, "Reconstruction algorithm for single-particle diffraction imaging experiments", Phys Rev E 80(2) (2009).
[2] H. T. Philipp, K. Ayyer, M. W. Tate, V. Elser and S. M. Gruner, "Solving structure with sparse, randomly-oriented x-ray data", Opt Express 20(12), 13129-13137 (2012).
[3] Kartik Ayyer, Hugh T. Philipp, Mark W. Tate, Jennifer L. Wierman, Veit Elser, and Sol M. Gruner, "Determination of crystallographic intensities from sparse data," IUCrJ 2(1), 29-34 (2015).
[4] K. Ayyer, H. T. Philipp, M. W. Tate, V. Elser and S. M. Gruner, "Real-Space x-ray tomographic reconstruction of randomly oriented objects with sparse data frames", Opt Express 22(3), 2403-2413 (2014).
Submitted by: Ernest Fontes, CHESS, Cornell University
01/13/2015
