X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18
Canine parvovirus (CPV) is closely related to the feline panleukopenia virus (FPV), also a parvovirus, that infects domestic cats and some non-domestic carnivores. In the 1970s, canine parvovirus type 2 (CPV-2) came to the scene, and had spread around the world by 1978 to be almost completely replaced by a mutant CPV-2a variant by the end of the eighties. The CPV-2a variant has a broad host range infecting both domestic and wild carnivores (incl. dogs and cats). It’s been hypothesized that CPV-2a may be displacing FPV-like viruses in many wild carnivore hosts. Understanding the structural basis of virus-host recognition is therefore of utmost importance to be able to design strategies for intercepting infections with this high-fatality rate virus.
The group of scientists led by Prof. Susan Hafenstein of Pennsylvania State University were able to crystallize and solve the structure of the capsid of the CPV-2a variant (Figure 1, A). The 3.5Å crystal structure revealed many interesting details about the host interaction sites, giving new insights into why the CPV-2a variant has a range of hosts broader than its predecessor (CPV-2). The most significant structural difference between CPV-2 and CPV-2a is caused by an Ala300Gly mutation, which results in a 3Å movement of the GH loop and the loss of a stabilizing hydrogen bond interaction (Figure 1, B). This loop movement likely influences the binding between capsid and its major host cell receptor. Additionally, Gly300 is also within one of the major antigenic sites on the capsid.
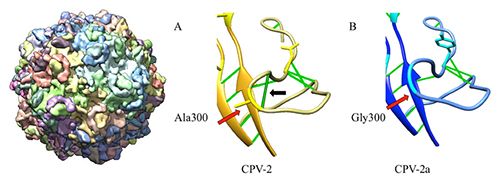
Figure 1: A) Capsid reconstruction of the CPV-2a variant; B) The 3Å GH loop movement is caused by a single point mutation from Ala to Gly.
In addition to others, one more noteworthy mutation for CPV-2a is the Asn to Aps change at positon 426. The Asp residue introduces a negative charge that prevents the interaction of several of the antigenic site monoclonal antibodies. The seemingly minor rearrangements and subtle conformational changes that enable enhanced flexibility of the capsid have had a profound impact on the success of the virus in nature and its ability to globally replace CPV-2. The findings were published in the Journal of Virology.
X-ray diffraction data was collected at the F1 beamline at CHESS.
Reference:
[1] Organtini LJ, Allison AB, Lukk T, Parrish CR, Hafenstein S, "Global displacement of canine parvovirus by a host-adapted variant: structural comparison between pandemic viruses with distinct host ranges", J Virol. 2015 Feb; 89(3):1909-12.
Submitted by: Tiit Lukk, MacCHESS, Cornell University
05/29/2015
