X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18

MacCHESS post-doc TK Chua seeks to understand the operation of enzymes that operate on gases, such as carbonic anhydrase and nitric oxide synthase, by applying the pressure-cryocooling technique to trap gas molecules in their active sites.
Pressure cryocooling, originally developed by Chae Un Kim and Sol Gruner (1) and now supported as a resource by MacCHESS, has seen most use as a means of reducing the damage caused to macromolecular crystals when they are cooled to 100 K. By pressurizing crystals with helium gas at about 2 kbar before and during cooling, it is often possible to maintain the original, pre-cooling, crystal quality. Moreover, it is usually not necessary to add cryoprotectants (such as glycerol, sucrose, or polyethylene glycol) to the crystal to prevent ice formation; the high pressure takes care of this. MacCHESS offers a pressure-cryocooling service to users whose crystals suffer damage during cryocooling, and a number of successes have been recorded.
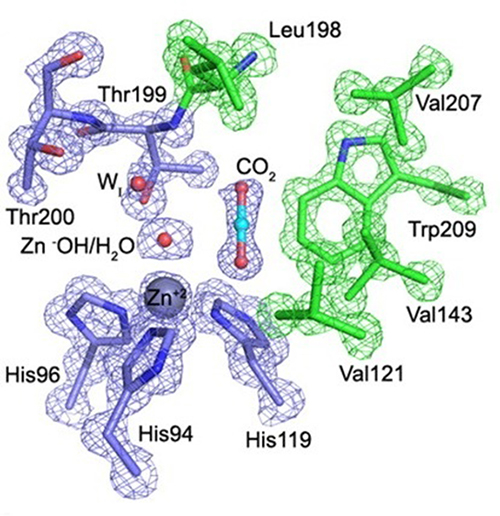
Figure 1
In addition to improved cryocooling, application of pressure can change equilibria in a crystal, leading to the trapping of states that could not otherwise be observed. TK Chua, a post-doc from Singapore who joined MacCHESS in October 2014, is focusing on the trapping of gases in enzymes. In this process, helium is replaced by the gas to be trapped, and the pressure applied only needs to be high enough to ensure that the active site of the enzyme is fully occupied by the gas. The first system TK worked on, in collaboration with Rob McKenna from U. Florida and Mayank Aggarwal of Oak Ridge, was carbonic anhydrase (CA). CA is an enzyme that converts carbon dioxide to bicarbonate; in humans this acts to regulate the pH of the blood, and in industry a potential CO2 sequestrator. In a previous collaboration between McKenna and Kim, the structure of human CA II (hCA) loaded with CO2 was determined (2); see Figure 1. Recently, Aggarwal provided TK with a β-CA from the bacterium Pseudomonas aeruginosa (bCA). TK grew crystals, pressurized them with CO2 at ~10 atmospheres, and cooled them to liquid nitrogen temperature under pressure. Then he collected data and solved the crystal structure. The placement of CO2 relative to the catalytic zinc ion and critical water molecules is quite similar in hCA and bCA, in spite of completely different active site structure in the human and bacterial enzymes (Figure 2). A paper comparing the structures in detail has been submitted.
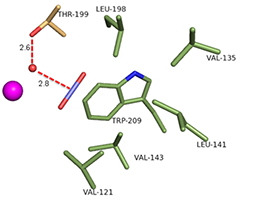
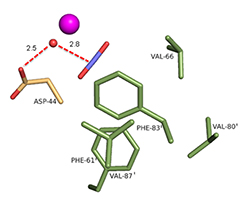
Figure 2: CO2 in the active site of (Left) hCA II, and (Right) bCA. Residues are as labeled. Similar CO2 binding orientation relative to the zinc-bound solvent.
For his next project, TK is collaborating with Brian Crane of Cornell, attempting to trap NO in nitric oxide synthase (NOS). This system has not previously been studied using pressure cryocooling, and there is a complex set of factors to consider, including selection of an appropriate species for study, crystallization conditions, whether to add co-factors, etc. It's a work in progress. TK is also helping to upgrade the MacCHESS pressure-cryocooling apparatus, following the lead of the Carpentier group at ESRF (3); these upgrades should both extend the capabilities of the apparatus and make it easier to use.
References:
[1] Kim, C. U.; Kapfer, R.; Gruner, S. M. Acta Crystallogr. D61, 881–890 (2005).
[2] Domsic, J. F.; Avvaru, B. S.; Kim, C. U.; Gruner, S. M.; Agbandje-McKenna, M.; Silverman, D. N.; McKenna, R. J. Biol. Chem. 283, 30766-71 (2008).
[3] Van der Linden, P.; Dobias, F.; Vitoux, H.; Kapp, U.; Jacobs, J.; McSweeney, S.; Mueller-Dieckmann, C.; Carpentier, P. J. Appl. Crystallogr. 47, 584-592 (2014).
Submitted by: Marian Szebenyi, MacCHESS, Cornell University
07/31/2015
