X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18
A key element for bringing organic electronics to the market place is the understanding of the microstructure formation during coating and printing. The deposit microstructure, i.e. grain orientation and grain boundaries, limits the device performance such as mobility and quantum efficiency. In a recent paper published in APL Materials [1], Randy Headrick and coworkers at the University of Vermont studied the structure formation of the solvable organic semiconductor C8-BTBT. Using their pen writing stage and the microbeam grazing-incidence wide-angle scattering (µGISAXS) set-up at CHESS D1 station, graduate students Jing Wan and Yang Li, with the assistance of beamline scientist Detlef Smilgies, performed coating experiments with coating speeds up to 25 mm/s (or 1.5 m/min) which is approaching relevance to industrial production.
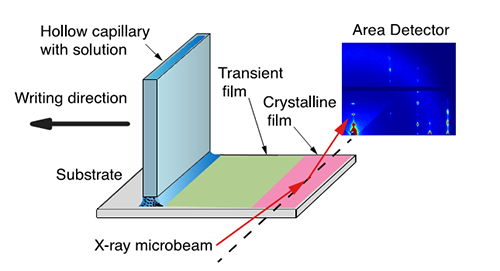
Figure 1: Experimental set-up at D1 station with incident microbeam, penwriter, and fast x-ray detector. The detector image shows the onset of crystallization with highly oriented crystallites, and the solvent ring of the evaporating solution.
The pen writer consists of a glass capillary that is moved along the substrate with a programmable speed motor. Due to capillary action the C8-BTBT solution is deposited on the substrate surface. A Pilatus 200k pixel array detector captures up to 100 frames per second of the scattered x-rays from the drying film providing x-ray movies of the film during coating and drying. At a speed of 25 mm/s deposition happens on a faster time scale than evaporation, and a liquid film is coated which dries successively. The Vermont group found in lab experiments that such films have a good mobility, and wanted to characterize the crystallization in detail using synchrotron radiation.
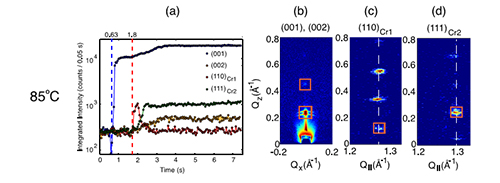
Figure 2: Intensities of characteristic reflections of the LC phase (001) and the two crystalline phases Cr1 and Cr2. The corresponding detector images for the reflections are shown in panels (b) through (d).
The findings were unexpected. Figure 2 shows that at first only the (001) reflection is visible but no other reflections which was ascribed to the formation of a smectic liquid-crystalline (LC) phase. After a temperature-dependent time delay, crystallization sets in, first a metastable structure (Cr1), then the final equilibrium thin film structure (Cr2). Above 95°C the films remain in the LC phase. Despite this complex crystallization process the final structure is highly oriented. Lab-based cross-polarized microscopy shows spherulites with millimeter-scale grains. The experiment demonstrates, that even at this high coating speed — the highest reported so far — high-quality films can form.
References:
[1] Jing Wan, Yang Li, Jeffrey G. Ulbrandt, Detlef-M. Smilgies, Jonathan Hollin, Adam C. Whalley, and Randall L. Headrick: "Transient phases during fast crystallization of organic thin films from solution", APL Mater. 4, 016103 (2016).
[2] Songtao Wo, Randall L. Headrick, and John E. Anthony: "Fabrication and characterization of controllable grain boundary arrays in solution-processed small molecule organic semiconductor films", J. Appl. Phys. 111, 073716 (2012).
Submitted by: Detlef Smilgies, CHESS, Cornell University
01/07/2016
