X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18
There has been much recent excitement about the use of nanometer-sized crystals (NC) for a wide variety of applications ranging from optoelectronics to catalysis to biology and medicine. Nanocrystals are formed by an inorganic core, which can be a metal, and insulator, or, as in the case of lead sulfide (PbS), a semiconductor. Nanocrystals may be synthesized with a corona of organic molecules anchored on the nanocrystal surface. These so-called ligands, typically alkyl chains with a reactive headgroup for the anchoring, facilitate dissolving the nanocrystals in suitable solvents. This eases processibility, which is important for the wet-chemical fabrication of organic solar cells and many other applications.
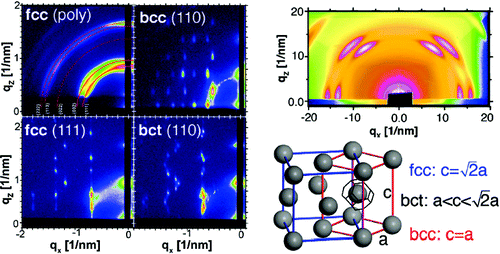
Solvent vapor controlled nanocrystal self-assembly. The composite figure on the left shows GISAXS images for different superlattices formed by PbS nanocrystals at different hexane vapor pressures. Top right: GIWAXS scattering from the atomic lattice of the PbS cores in the BCC superlattice. Bright spots indicate that individual nanocrystals have a specific orientation relative to the superlattice. Bottom right: Bain path from an FCC lattice to a BCC lattice via continuous BCT phases by shrinking of the c/a ratio.
When we think of a crystal, say a diamond, we think of it as something hard and unchangeable in which every atom sits at its prescribed place. Nanocrystals can themselves be organized into a periodic lattice, thereby forming a superlattice, i.e., a crystal of nanocrystals. Recently a group of researchers at Cornell University and the Cornell High Energy Synchrotron Source (CHESS) studied what happens to nanocrystal superlattices, if they are immersed in the vapor of a good solvent. Chemical engineering professor Tobias Hanrath and his grad students Kaifu Bian (Chemical & Biological Engineering) and Josh Choi (Applied & Engineering Physics) synthesized high-quality PbS nanocrystals. They worked at CHESS D-line with staff scientist Detlef Smilgies who has developed a sample cell in which nanocrystal deposits can be kept under a controlled solvent vapor pressure. Both grazing incident small-angle x-ray scattering (GISAXS) and grazing incident wide-angle x-ray scattering (GIWAXS) can be performed while the deposit is immersed in solvent vapor. GISAXS provides a very precise characterization of superlattice symmetry and orientation – many nanocrystal superlattices favor close-packed planes parallel to the substrate. GIWAXS probes the scattering from individual nanocrystals. Since superlattices can be coaxed by vapor processing into high order and orientation, GIWAXS is able to detect the orientation of individual nanocrystals on their superlattice sites. The correlation between superlattice symmetry and nanocrystal orientation is the main result of the paper.
In the course of their studies the researchers found that nanocrystals initially formed a face-centered cubic (FCC) lattice which is well-known to yield the densest packing of spheres in 3 dimensions. Upon treating the deposits with solvent vapor, the neat x-ray diffraction patterns of this high-symmetry structure began to split up forming much more complex spot patterns of the scattered intensity. These complicated images could be ascribed to a lattice distortion: one of the cubic axes shrinks relative to the other 2, and a body-centered tetragonal (BCT) lattice is formed. Eventually the diffraction pattern becomes neater again with fewer spots of bright intensity. The new high-symmetry lattice is body-centered cubic (BCC). This type of transition, in which one axis continuously deforms, is well-known in certain atomic lattices undergoing a martensitic transition with temperature. The classic example is iron, which is BCC at room temperature and FCC at elevated temperatures. Hanrath’s study showed for the first time such a Bain transition in ordered assemblies of nanocrystals of just one type of particle.
Just observing the transition did not provide the clue, what the underlying driving force was. So Hanrath and coworkers invited theorist Paulette Clancy (Chemical Engineering) and her postdoc Ananth Kaushik to join forces with them. Clancy and Kaushik studied the interaction of 2 nanocrystals via their ligand shells and found evidence for a mechanism that coordinated particles. The effect was confirmed by GIWAXS measurements that showed that the PbS particles which have the shape of truncated octahedra have a specific orientation relative to each other as well as to the superlattice as a whole in the BCC phase, while they have a random orientation in the FCC lattice, as ideal spheres would show. The BCT phases show an increasing degree of orientation until the BCC symmetry is attained. Hence the underlying force was identified as the ligand-mediated shape anisotropy of the particles.
Hanrath's work was highlighted by an ACS Nano podcast, episode 45 (http://pubs.acs.org/page/ancac3/audio/index.html), as one of 3 papers in the April 2011 episode. Hear Tobias Hanrath and Detlef Smilgies talk about the subtleties of nanocrystal interactions, the role of solvent vapor, and the importance of in-situ and real time scattering experiments to elucidate the mechanisms of superlattice formation. In addition, renowned nanocrystal pioneer Brian Korgel and his student Brian Goodfellow, both also avid CHESS users, wrote a Perspective, that puts Hanrath’s work in context with the early nanocrystal work in the mid 90’s and today’s booming field. They also drew some fascinating parallels between nanocrystal self-assembly and microphase separation in block copolymers. Many bits and pieces of the puzzle are known, but a comprehensive theory that will predict how a given kind of nanocrystals will assemble under given conditions is still lacking. It is the hope of ongoing experimental and theoretical work at Cornell and other places, to get a step closer.
see also Highlight "Nanocrystal Arrays for
Optoelectronics":
http://news.chess.cornell.edu/articles/2011/Woll040511.html
References:
Kaifu Bian, Joshua J. Choi, Ananth Kaushik, Paulette Clancy, Detlef-M. Smilgies, and Tobias Hanrath; "Shape-Anisotropy Driven Symmetry Transformations in Nanocrystal Superlattice Polymorphs", ACS Nano 5, 2815–2823 (2011)
Brian W. Goodfellow and Brian A. Korgel; "Reversible Solvent Vapor-Mediated Phase Changes in Nanocrystal Superlattices", ACS Nano 5, 2419–2424 (2011)
Submitted by: Detlef Smilgies, CHESS, Cornell University
