X-RAY RUNS: Apply for Beamtime
2017 Nov 1 - Dec 21
2018 Feb 7 - Apr 3
2018 Proposal/BTR deadline: 12/1/17
2018 Apr 11 - Jun 4
2018 Proposal/BTR deadline: 2/1/18
Imagine an octahedron inside a cube and then let’s put spheres on the corner of the cube. Now the octahedron is well enclosed inside the cube and also well-separated from octahedra in adjacent cubic unit cells. The octahedron itself can be composed of smaller particles located at the corners of the octahedron. Such binary structure of large iron oxide spheres of 13 nm diameter enclosing a hexamer of six small 4nm lead sulfide particles is known as an AB6 binary superlattice.
In a recent Scientific Report [1] by a collaboration of Cornell researchers, such AB6 structures were synthesized and characterized with grazing-incidence small-angle x-ray scattering and electron microcopy. Moreover, the high-quality superlattices gave rise to multiple superlattice Bragg reflections so that a crystal structure determination could be performed based on small-angle scattering data. The distance between the lead sulfide particles of the central hexamer is encoded in the scattering intensities, and hence a full simulation of the nanoparticle scattering pattern became necessary.
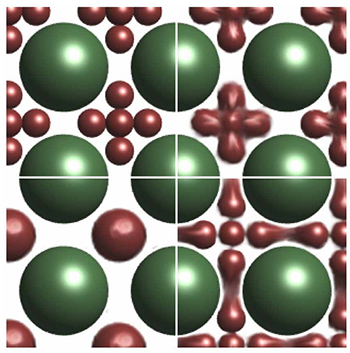
Figure 1. Different stages of fusion of the center lead sulfide hexamer (brown) in the iron oxide (green) cages as a function of temperature.
Arriving at this point, another experiment could be introduced, a heat treatment of the superlattices. It has been previously reported that nanoparticles can sinter or fuse [2,3] when heated. This is facilitated by the strong reduction in melting temperature typically seen in nanocrystals; hence nanoparticles can fuse at temperatures between 100°C and 200°C while the organic ligand molecules are still stable. Says team leader Tobias Hanrath, a professor at the Cornell School of Chemical and Biomolecular Engineering: “The question was whether the whole binary superlattice would just clump together, or whether something more interesting would happen, due to the large size difference between lead sulfide and iron oxide nanoparticles, and thus their melting points.”
Graduate student Ben Treml of the Hanrath group synthesized the particles and self-assembled them into superlattices using the inclined-plane drying method commonly used for colloidal superstructures. The samples were characterized at CHESS D1 station with the help of CHESS staff scientist Detlef Smilgies. Treml and Smilgies also collaborated on the crystal structure analysis of the binary superlattices based on a method developed by Smilgies and coworkers earlier [4,5]. The AB6 superlattice reflections survived temperatures up to 200°C. Moreover, nine well-defined Bragg reflections showed relative intensity changes at different stages of the heat treatment. The crystal structure analysis revealed that the particles forming the octahedron approached each other as a function of the annealing temperature and eventually merged into a single particles, changing the lattice symmetry to the cesium chloride structure AB. As an alternative thermal annealing route, Treml used laser spike annealing in Professor Mike Thompson’s lab at Cornell Materials Science and Engineering, and found that at a similar stage of approach of the hexamer particles, the laser-annealed samples yielded superlattices with a lower degree of disorder.
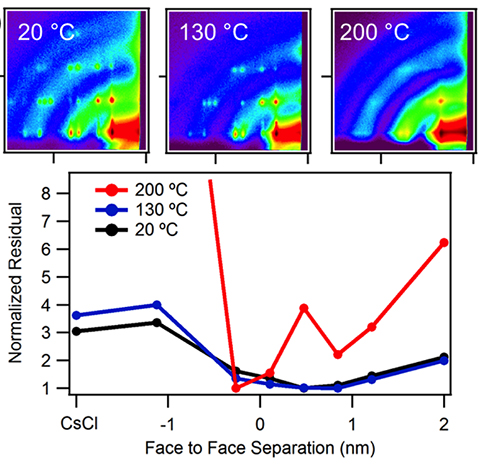
Figure 2. GISAXS images of AB6 binary nanocrystal superlattices annealed at different temperatures, and the reduction of particle separation in the lead sulfide hexamer based on the structure analysis.
Treml and Hanrath approached their chemical engineering colleague Professor Paulette Clancy and her post-doctoral student Binit Lukose about theoretical modelling of the process for further support and understanding of the structural results. Molecular dynamics calculations ruled out the possibility of some structures (e.g., cuboctahedra) and confirmed that a truncated octahedron was the most likely option. It also revealed that there are two types of cavities, one inside the hexamer where the oleic acid ligands on the lead sulfide hexamer interdigitate, facilitating the approach of inorganic cores towards each other to eventually fuse. Ligands on the outside of the hexamer stretch out towards the surrounding iron nanocrystals. It became clear that a reduction of ligand density would be needed in order to accommodate the small interparticle spacings obtained from the structure analysis.
The demonstration of a controlled chemical reaction inside a cage of large and relatively inert nanoparticles may translate to other reactions and materials. The self-assembly process guarantees that particles are located in each cubic cage and thus are well intermixed. This route avoids the problem of filling nanoporous voids with reactants as encountered in zeolites.
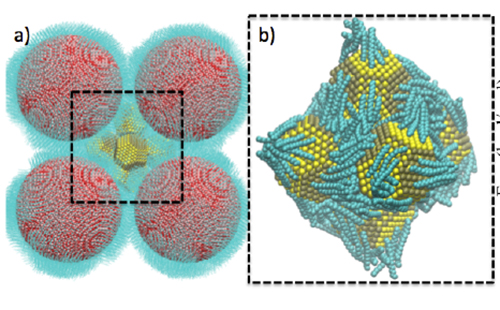
Figure 3. Molecular Dynamics-generated snapshots of the system. a) shows the surrounding Fe2O3 spheres (red) enclosing a hexamer of PbS nanocrystals (yellow). b) Zooms in on the PbS hexamer to show the disposition of the oleic acid ligands (turquoise) at a surface ligand density of 1.1 nm-2.
References:
[1] Benjamin E. Treml, Binit Lukose, Paulette Clancy, Detlef-M Smilgies, and Tobias Hanrath: "Connecting the Particles in the Box -Controlled Fusion of Hexamer Nanocrystal Clusters within an AB6 Binary Nanocrystal Superlattice", Scientific Reports 4, 6731 (2004) (DOI: 10.1038/srep06731).
[2] Brian Goodfellow, Reken Patel, Matthew Panthani, Detlef-M. Smilgies, and Brian Korgel: "Melting and Sintering of a Body-Centered Cubic Superlattice of PbSe Nanocrystals Followed by Small Angle X-ray Scattering", J. Phys. Chem. C 115, 6397–6404 (2011).
[3] Brian W. Goodfellow, Michael R. Rasch, Colin M. Hessel, Reken N. Patel, Detlef-M. Smilgies, and Brian A. Korgel: "Ordered Structure Rearrangements In Heated Gold Nanocrystal Superlattices", Nano Lett. 13, 5710–5714 (2013). (see CHESS News
http://news.chess.cornell.edu/articles/2013/KorgelFused11012013.html)
[4] Andrew T. Heitsch, Reken N. Patel, Brian W. Goodfellow, Detlef-M. Smilgies, and Brian A. Korgel: "GISAXS Characterization of Order in Hexagonal Monolayers of FePt Nanocrystals", J. Phys. Chem. C 114, 14427–14432 (2010).
[5] Detlef-M. Smilgies, Andrew T. Heitsch, and Brian A. Korgel: "Stacking of Hexagonal Nanocrystal Layers During Langmuir-Blodgett Deposition", J. Phys. Chem. B 116, 6017-6026 (2012).
Submitted by: Detlef Smilgies and Paulette Clancy
CHESS, Cornell University
10/25/2014
